Impact of Transgenic Maize Ruifeng125 on Diversity and Dynamics of Bacterial Community in Rhizosphere Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Test Design
2.3. Collection of Rhizosphere Soil
2.4. DNA Extraction and PCR Amplification
2.5. Detection of Bt Protein Contents in the Rhizosphere Soil
2.6. Illumina Miseq Sequencing and Data Processing
3. Results
3.1. The Accumulation and Degradation Dynamics of Bt Protein in the Rhizosphere Soil
3.2. Statistics and Analysis of Sequencing Data
3.3. Changes of Bacterial Community Structure in Rhizosphere Soils of Transgenic Maize Ruifeng125 and Its Control Line
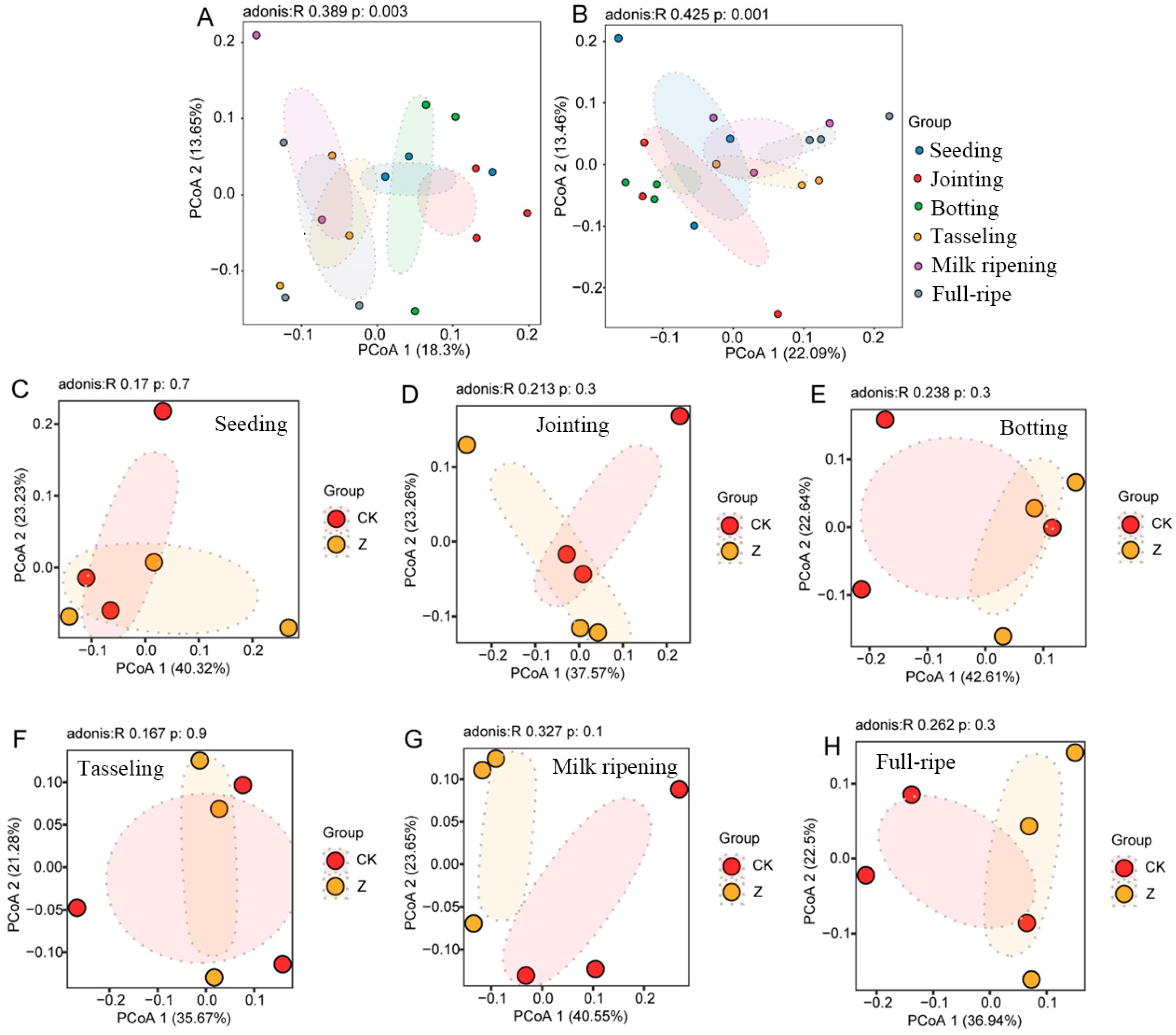
3.4. Beta Diversity of Rhizosphere Bacterial Community of the Two Maize Lines
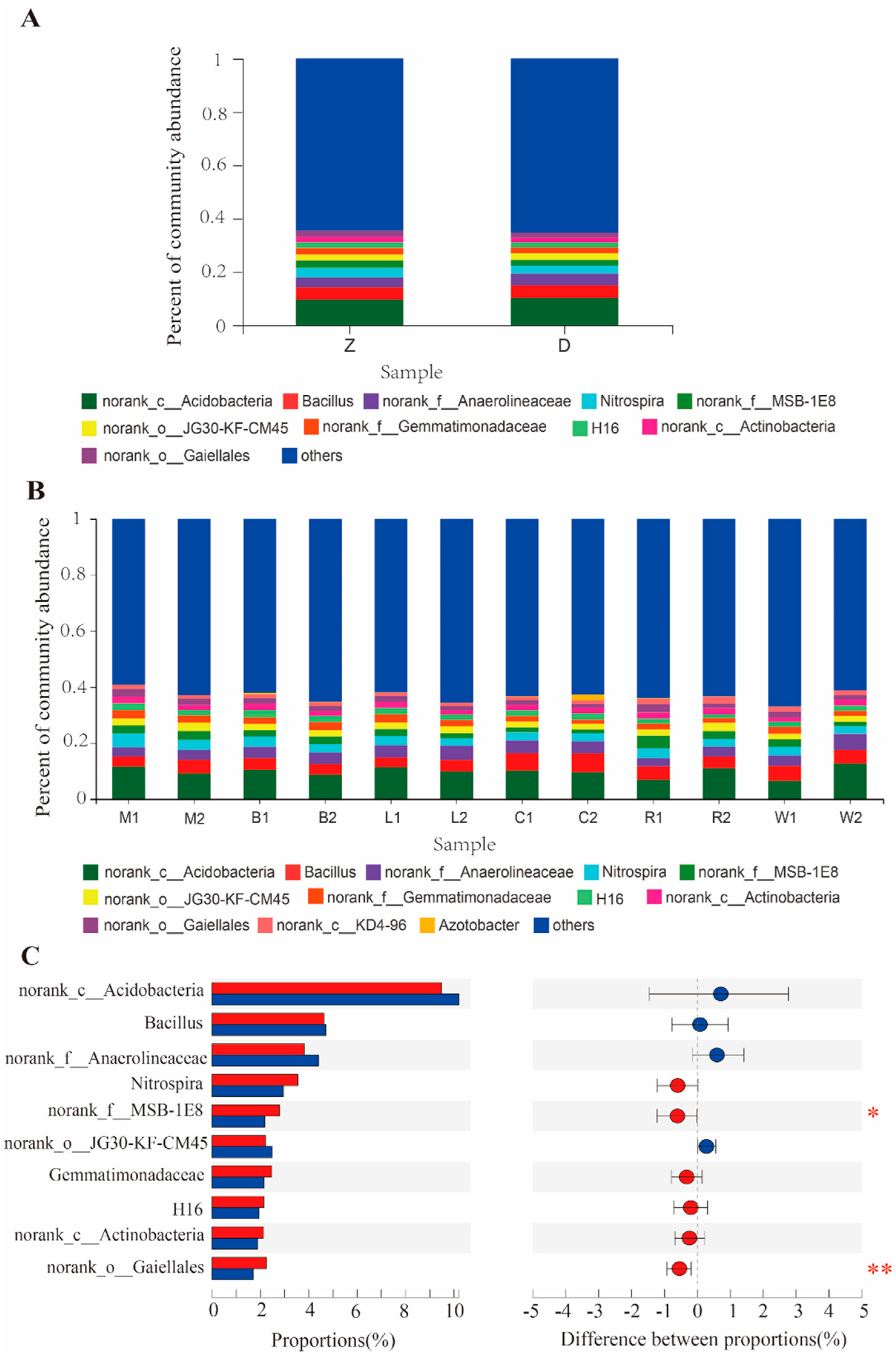
3.5. Difference in Bacterial Community Abundance in Two Rhizosphere Soils
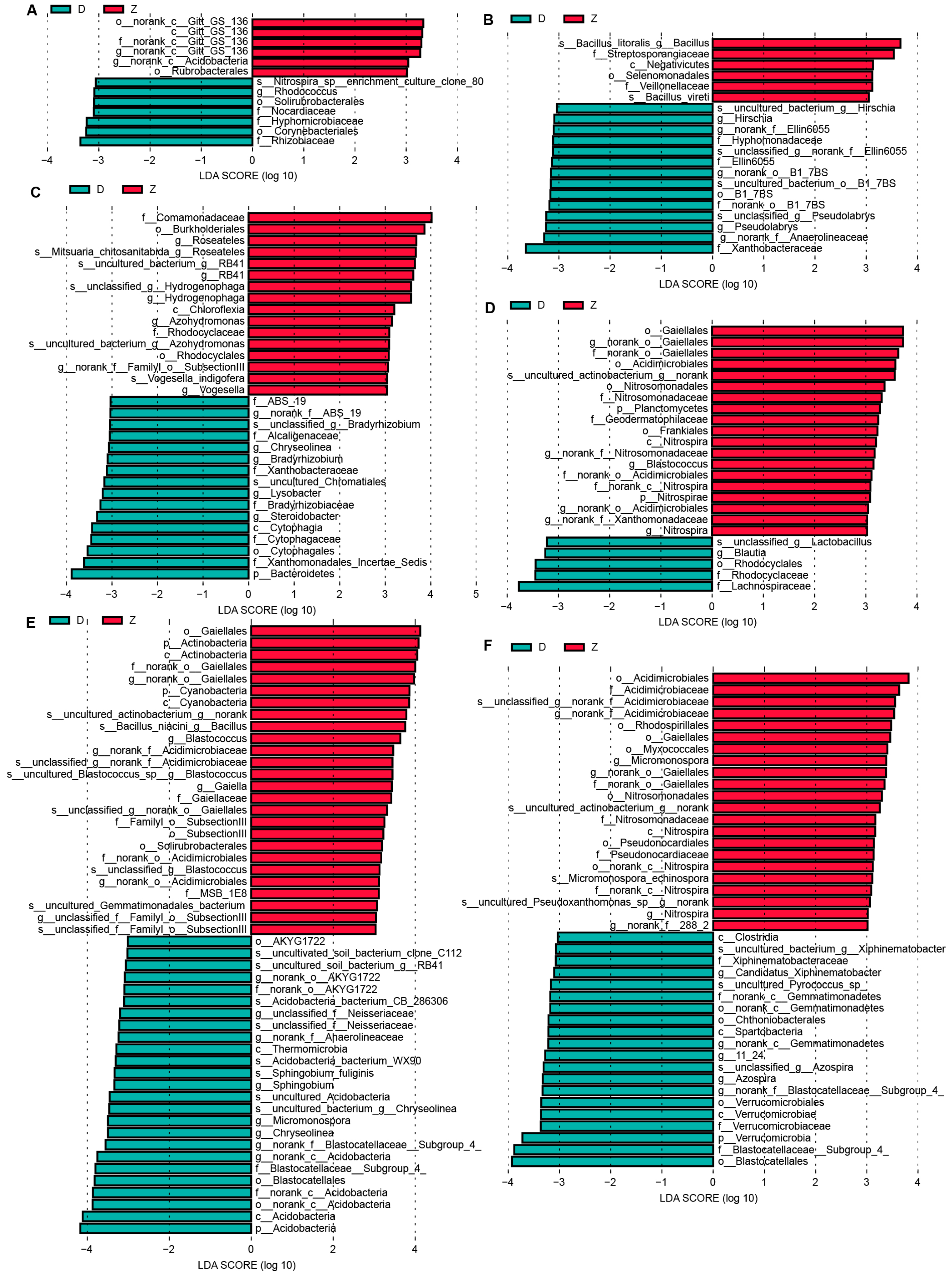
3.6. Biomarkers between Transgenic Maize Ruifeng125 and Non-Transgenic Control along the Six Developmental Stages
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Conner, A.J.; Glare, T.R.; Nap, J.P. The release of genetically modified crops into the environment. Plant J. 2003, 33, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Qaim, M.; Zilberman, D. Yield Effects of Genetically Modified Crops in Developing Countries. Science 2003, 299, 900–902. [Google Scholar] [CrossRef]
- Campos, S.O.; Santana, I.V.; Silva, C.; Santos-Amaya, O.F.; Guedes, R.N.C.; Pereira, E.J.G. Bt-induced hormesis in Bt-resistant insects: Theoretical possibility or factual concern? Ecotoxicol. Environ. Saf. 2019, 183, 109577. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Qi, L.; Chen, F.; Liu, M.; Whalen, J.K. Root and detritus of transgenic Bt crop did not change nematode abundance and community composition but enhanced trophic connections. Sci. Total Environ. 2018, 644, 822–829. [Google Scholar] [CrossRef]
- Cheng, M.M.; Shu, Y.H.; Wang, J.W. Effect of Bt rice straw returning in soil on the growth and reproduction of Eisenia fetida. Ying Yong Sheng Tai Xue Bao 2016, 27, 3667–3674. [Google Scholar] [PubMed]
- Zhang, W.; Cao, Z.; Wang, M.; Chen, X.; Wang, B. Absorption, translocation, and effects of Bt Cry1Ac peptides from transgenic cotton to the intercrops and soil functional bacteria. Sci. Rep. 2020, 10, 17294. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chang, L.; Reddy, G.V.P.; Zhang, L.; Fan, C.; Wang, B. Use of taxonomic and trait-based approaches to evaluate the effects of transgenic Cry1Ac corn on the community characteristics of soil collembola. Environ. Entomol. 2019, 48, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wu, F.; Dong, J.; Wang, B.; Yin, J.; Song, X. No impact of transgenic cry1Ie maize on the diversity, abundance and composition of soil fauna in a 2-year field trial. Sci. Rep. 2019, 9, 10333. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Chernet, M.T.; Tuji, F.A. Phenotypic, stress tolerance, and plant growth promoting characteristics of rhizobial isolates of grass pea. Int. Microbiol. 2020, 23, 607–618. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; Song, P.Y.; Ping, Y.S.; Yong, L.I. Correlation between soil microbes and plant diversity under the typical subtropical natural succession forest. J. Southwest Univ. (Nat. Sci. Ed.) 2014, 36, 129–134. [Google Scholar]
- He, Z.L. Soil microbial biomass and its significance in nutrient cycling and environmental quality assessment. Soil 1997, 2, 61–69. [Google Scholar]
- Zhou, L.X.; Ding, M.M. Soil microbial characteristics as bioindicators of soil health. Biodivers. Sci. 2007, 2, 162–171. [Google Scholar]
- Wang, X.P.; Yang, X.; Yang, N.; Xin, N.; Qu, Y.; Nianxi, Z.; Gao, Y. Effects of litter diversity and composition on litter decomposition characteristics and soil microbial community: Under the conditions of doubling ambient atmospheric CO2 concentration. Ecol. Sci. 2019, 39, 6264–6272. [Google Scholar]
- Li, X.G. Effect of Transgenic Insect-Resistant Cotton on Soil Ecosystem; Nanjing Forestry University: Nanjing, China, 2011. [Google Scholar]
- Xing, Z.J.; Wang, Z.Y.; He, K.L.; Bai, S.X. Degradation dynamics of Cry1Ab insecticidal protein expression in transgenic Bacillus thuringiensis corn plants under different conditions and its accumulation in soil. In Proceedings of the Annual Meeting of the China Association for Science and Technology, Beijing, China, 16–20 September 2006. [Google Scholar]
- Chen, Q. Hybridization between Fagopyrum (Polygonaceae) species native to China. Bot. J. Linn. Soc. 1999, 131, 177–185. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.W.; Wu, H.-B.; Yang, C.L.; Li, B.D.; Lu, X.B. Quantity of culturable microorganism and diversity of bacterial physiological groups in transgenic Bt corn rhizosphere. Chin. J. Ecol. 2010, 3, 101–106. [Google Scholar]
- Naef, A.; Zesiger, T.; Défago, G. Impact of transgenic Bt maize residues on the mycotoxigenic plant pathogen Fusarium graminearum and the biocontrol agent Trichoderma atroviride. J. Environ. Qual. 2006, 35, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.K.; Zhang, J.N.; Yang, D.L. Effects of transgenic Bt maize on bacterial community structure in rhizosphere soil. Chin. J. Ecol. 2011, 30, 98–105. [Google Scholar]
- Canfora, L.; Sbrana, C.; Avio, L.; Felici, B.; Scatà, M.C.; Neri, U.; Benedetti, A. Risk management tools and the case study Brassica napus: Evaluating possible effects of genetically modified plants on soil microbial diversity. Sci. Total Environ. 2014, 493, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ji, T.; Zhao, S.; Feng, H.; Wu, K. High-dose assessment of transgenic insect-resistant maize events against major lepidopteran pests in China. Plants 2022, 11, 3125. [Google Scholar] [CrossRef]
- Riley, D.; Barber, S.A. Salt Accumulation at the Soybean (Glycine Max. (L.) Merr.) Root-Soil Interface1. Soil Sci. Soc. Am. J. 1970, 34, 154–155. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Helassa, N.; M’charek, A.; Quiquampoix, H.; Noinville, S.; Déjardin, P.; Frutos, R.; Staunton, S. Effects of physicochemical interactions and microbial activity on the persistence of Cry1Aa Bt (Bacillus thuringiensis) toxin in soil. Soil Biol. Biochem. 2011, 43, 1089–1097. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Baum, C. The potential of rhizosphere microorganisms to promote the plant growth in disturbed soils. In Environmental Protection Strategies for Sustainable Development; Springer: Dordrecht, the Netherland, 2012; pp. 35–64. [Google Scholar]
- Liu, Y.; Li, J.; Luo, Z.; Wang, H.; Liu, F. The fate of fusion Cry1Ab/1Ac proteins from Bt-transgenic rice in soil and water. Ecotoxicol. Environ. Saf. 2016, 124, 455–459. [Google Scholar] [CrossRef]
- Singh, A.K.; Dubey, S.K. Current trends in Bt crops and their fate on associated microbial community dynamics: A review. Protoplasma 2016, 253, 663–681. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Ge, L.; Hu, C.; Wu, G.; Sun, Y.; Song, L.; Wu, X.; Pan, A.; Xu, Q. Environmental behaviors of Bacillus thuringiensis (Bt) insecticidal proteins and their effects on microbial ecology. Plants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Li, F.; Hao, C.; Sun, H.; Yang, S.; Jiao, Y.; Lu, X. Impact of insect-resistant transgenic maize 2a-7 on diversity and dynamics of bacterial communities in rhizosphere soil. Plants 2023, 12, 2046. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, M.; Xiao, L.; Song, X.; Yang, W.; Ding, G. Impact of water content and temperature on the degradation of Cry1Ac protein in leaves and buds of Bt cotton in the soil. PLoS ONE 2015, 10, e115240. [Google Scholar] [CrossRef] [PubMed]
- Jianwu, W.; Yuanjiao, F.; Shiming, L. Studies on spatial-temporal dynamics of insecticidal protein expression of Bt corn and its degradation in soil. Zhongguo Nongye Kexue 2003, 36, 1279–1286. [Google Scholar]
- Rui, Y.K.; Yi, G.X.; Zhao, J.; Wang, B.M.; Li, Z.H.; Zhai, Z.X.; He, Z.P.; Li, Q.X. Changes of Bt Toxin in the Rhizosphere of Transgenic Bt Cotton and its Influence on Soil Functional Bacteria. World J. Microbiol. Biotechnol. 2005, 21, 1279–1284. [Google Scholar] [CrossRef]
- Baumgarte, S.; Tebbe, C.C. Field studies on the environmental fate of the Cry1Ab Bt-toxin produced by transgenic maize (MON810) and its effect on bacterial communities in the maize rhizosphere. Mol. Ecol. 2005, 14, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
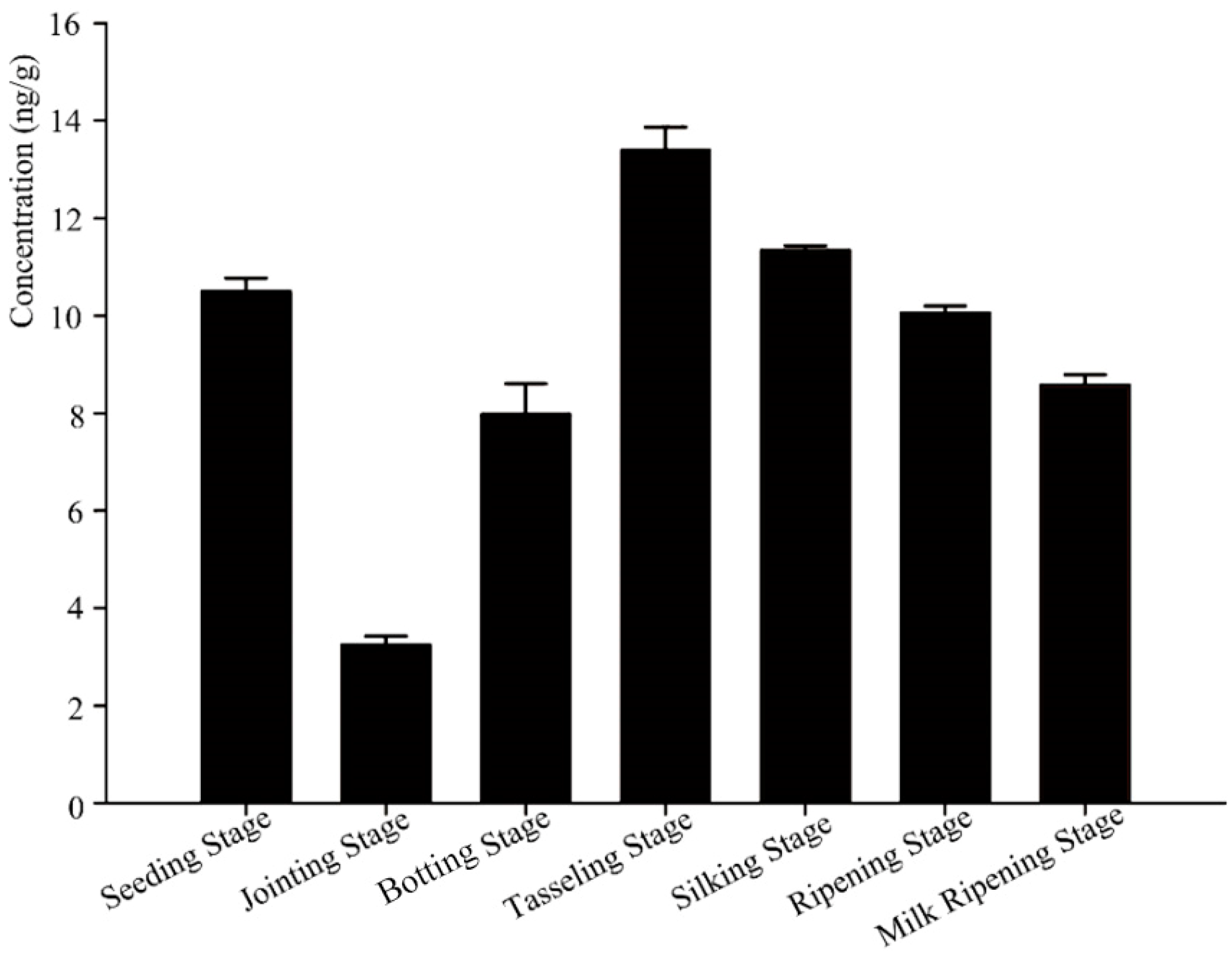



| Degragation (d) | Bt Protein Content (ng/g) | Protein Degradation Rate |
|---|---|---|
| 0 | 937.60 | 0 |
| 1 | 781.68 | 16.63% |
| 2 | 549.53 | 41.39% |
| 3 | 382.92 | 59.16% |
| 4 | 247.71 | 73.58% |
| 5 | 152.74 | 83.71% |
| 6 | 89.82 | 90.42% |
| 7 | 62.26 | 93.36% |
| 10 | 25.69 | 97.26% |
| 20 | 16.50 | 98.24% |
| 40 | 7.69 | 99.18% |
| 70 | 1.97 | 99.79% |
| 110 | 0.66 | 99.93% |
| 160 | 0.35 | 99.97% |
| 170 | -- | 100% |
| Group | Valid Reads | Average Length | Shannon Index | Chao Index | ACE Index | Simpson Index | Coverage |
|---|---|---|---|---|---|---|---|
| Z | 38,634 ± 4115 | 439 ± 0.75 | 6.99 ± 0.10 | 4157 ± 289 | 4160 ± 281 | 0.0024 ± 0.0003 | 0.96 ± 0.008 |
| D | 38,168 ± 4643 | 438.934 ± 0.79 | 7.00 ± 0.14 | 4051 ± 268 | 4070 ± 254 | 0.0025 ± 0.0012 | 0.96 ± 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, C.; Xia, X.; Xu, C.; Sun, H.; Li, F.; Yang, S.; Xu, X.; Lu, X. Impact of Transgenic Maize Ruifeng125 on Diversity and Dynamics of Bacterial Community in Rhizosphere Soil. Microorganisms 2024, 12, 1763. https://doi.org/10.3390/microorganisms12091763
Hao C, Xia X, Xu C, Sun H, Li F, Yang S, Xu X, Lu X. Impact of Transgenic Maize Ruifeng125 on Diversity and Dynamics of Bacterial Community in Rhizosphere Soil. Microorganisms. 2024; 12(9):1763. https://doi.org/10.3390/microorganisms12091763
Chicago/Turabian StyleHao, Chaofeng, Xinyao Xia, Chao Xu, Hongwei Sun, Fan Li, Shuke Yang, Xiaohui Xu, and Xingbo Lu. 2024. "Impact of Transgenic Maize Ruifeng125 on Diversity and Dynamics of Bacterial Community in Rhizosphere Soil" Microorganisms 12, no. 9: 1763. https://doi.org/10.3390/microorganisms12091763
APA StyleHao, C., Xia, X., Xu, C., Sun, H., Li, F., Yang, S., Xu, X., & Lu, X. (2024). Impact of Transgenic Maize Ruifeng125 on Diversity and Dynamics of Bacterial Community in Rhizosphere Soil. Microorganisms, 12(9), 1763. https://doi.org/10.3390/microorganisms12091763





