The Influence of Dietary Supplementation with Dried Olive Pulp on Gut Microbiota, Production Performance, Egg Quality Traits, and Health of Laying Hens
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Hens, Diets, and Experimental Design
2.3. Bird Productivity and Egg Quality
2.4. Blood Biochemical Parameters
2.5. Microbiota Analysis
2.5.1. Sample Collection and DNA Extraction
2.5.2. Sequencing Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Bird Productivity
3.2. Egg Quality Traits
3.3. Blood Biochemical Parameters
3.4. Dietary Effects of OP on Fecal Microbiome Structure
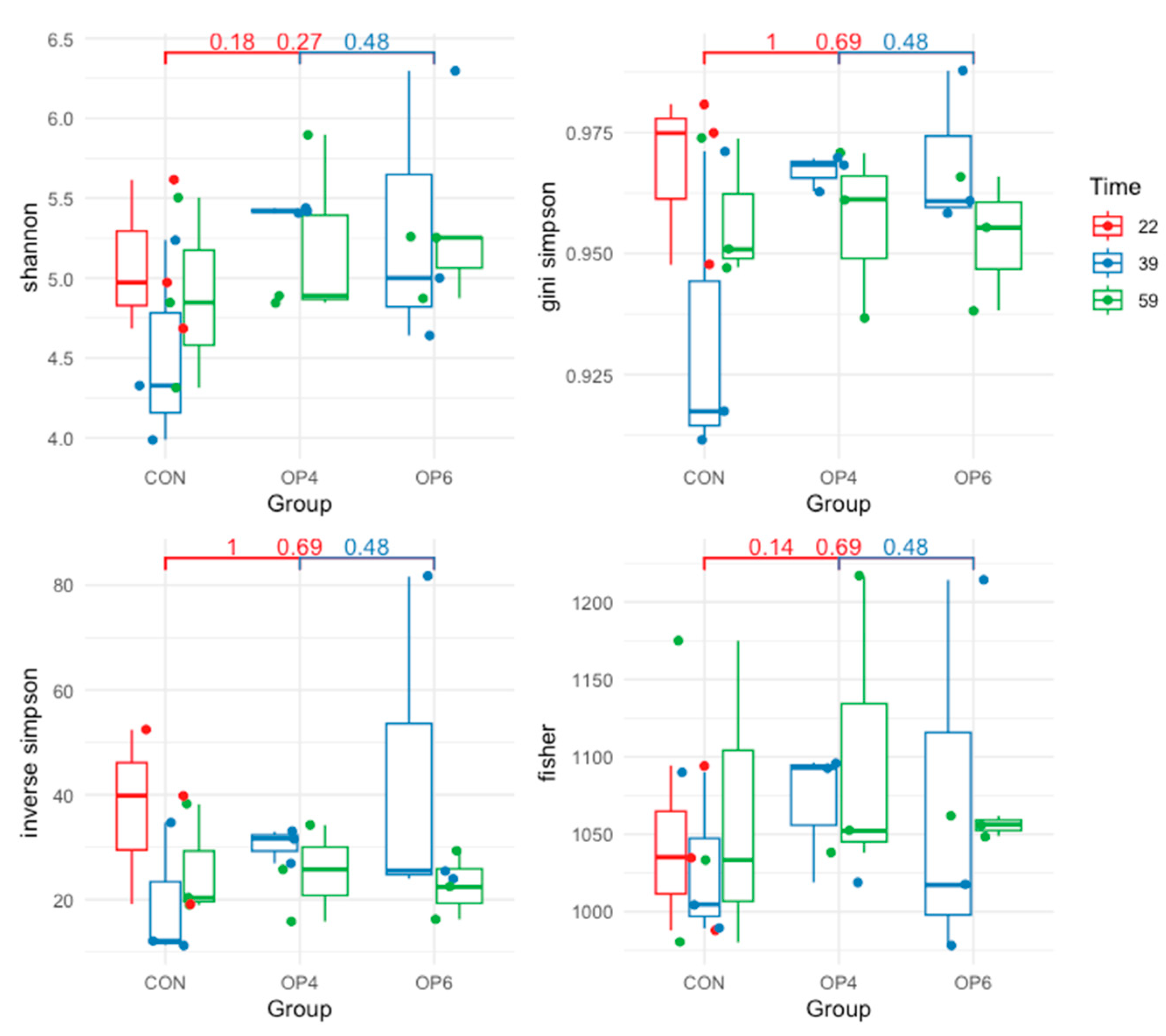
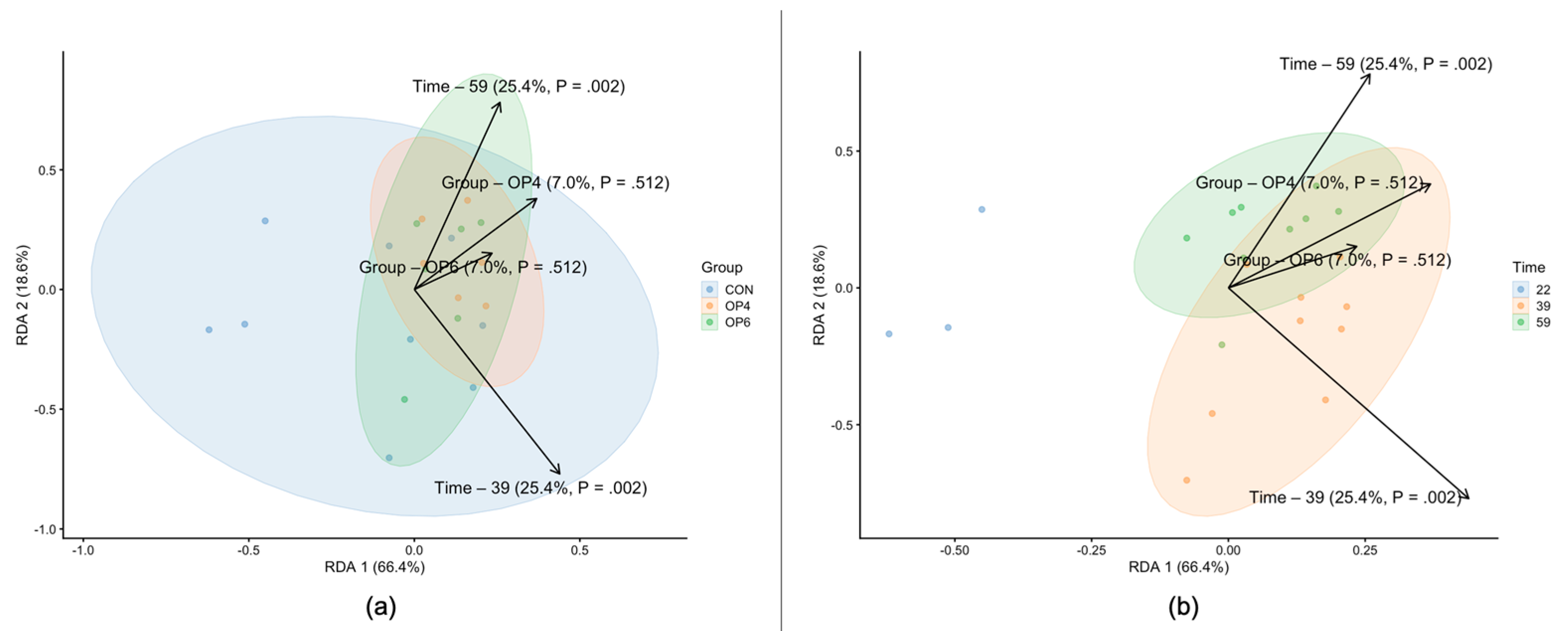
3.4.1. Microbiome Diversity Analysis
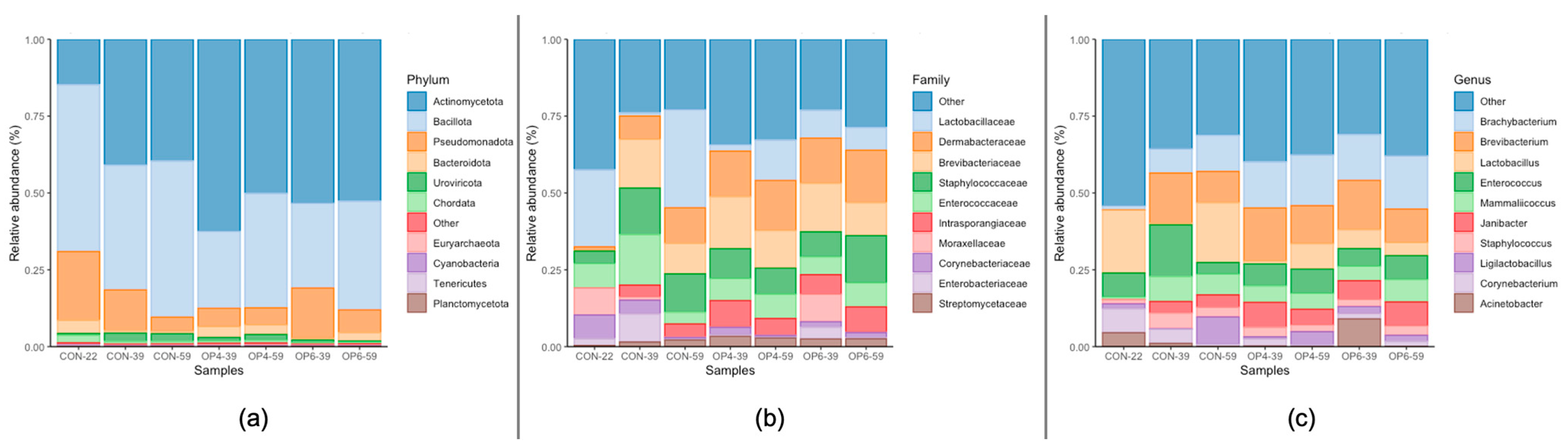
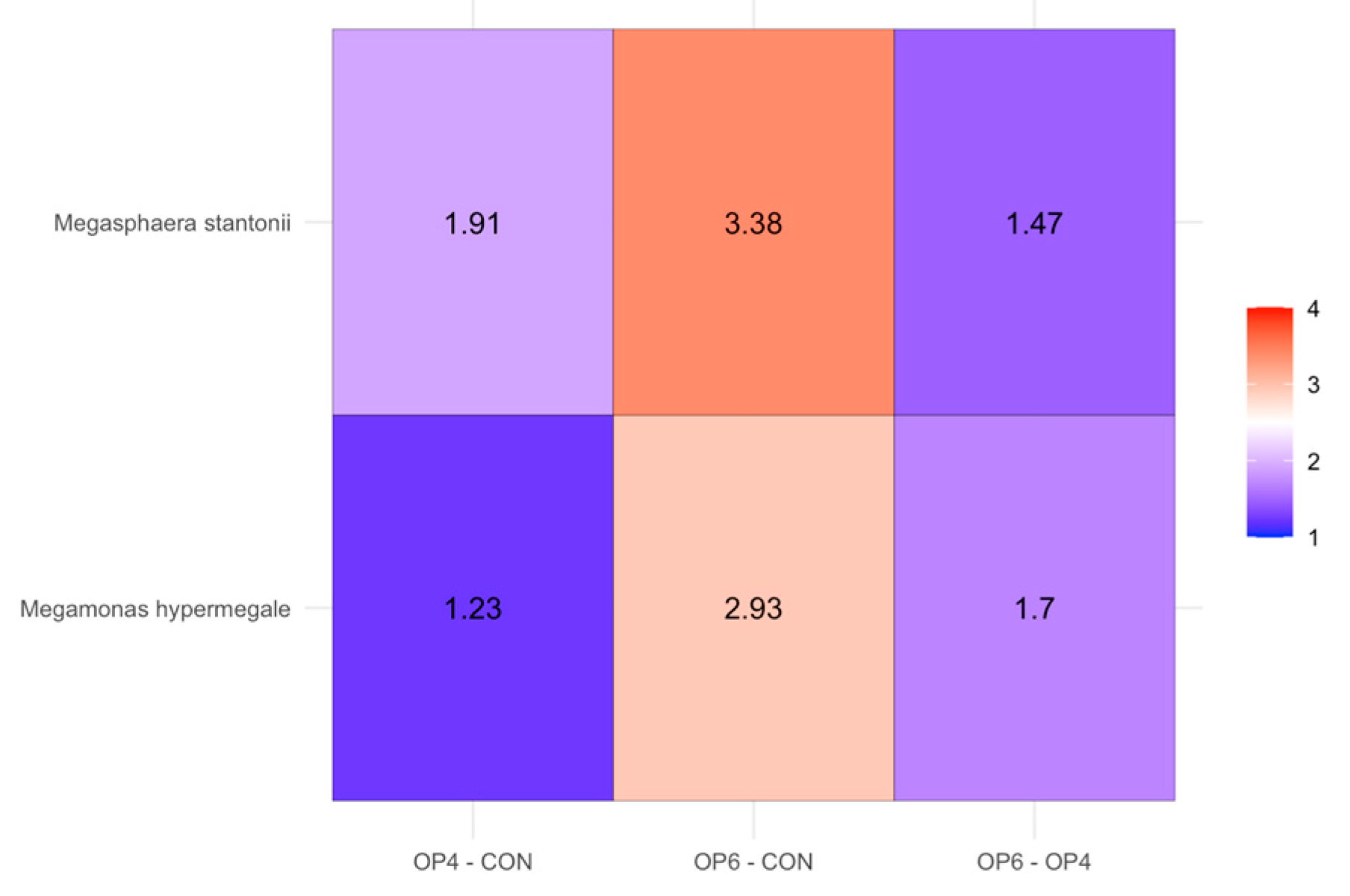
3.4.2. Microbial Community Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Eggs—Worldwide Statista Market Forecast. Available online: https://www.statista.com/outlook/cmo/food/dairy-products-eggs/eggs/worldwide (accessed on 30 January 2024).
- Molnár, A.; Kempen, I.; Sleeckx, N.; Zoons, J.; Maertens, L.; Ampe, B.; Buyse, J.; Delezie, E. Effects of Split Feeding on Performance, Egg Quality, and Bone Strength in Brown Laying Hens in Aviary System. J. Appl. Poult. Res. 2018, 27, 401–415. [Google Scholar] [CrossRef]
- Cheng, X.; Ning, Z. Research Progress on Bird Eggshell Quality Defects: A Review. Poult. Sci. 2023, 102, 102283. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Wang, J.; Wang, H.; Zhang, H.; Wu, S.; Qi, G. Dietary Tea Polyphenol Supplementation Improved Egg Production Performance, Albumen Quality, and Magnum Morphology of Hy-Line Brown Hens during the Late Laying Period1. J. Anim. Sci. 2018, 96, 225–235. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H.; Wu, S.; Qi, G.; Wang, J. Uterine Transcriptome Analysis Reveals mRNA Expression Changes Associated with the Ultrastructure Differences of Eggshell in Young and Aged Laying Hens. BMC Genom. 2020, 21, 770. [Google Scholar] [CrossRef]
- Yao, H.; Hu, Y.; Wang, Q.; Zhang, Y.; Rao, K.; Shi, S. Effects of Dietary Dimethylglycine Supplementation on Laying Performance, Egg Quality, and Tissue Index of Hens during Late Laying Period. Poult. Sci. 2021, 101, 101610. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics to Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Shang, H.; Zhao, J.; Dong, X.; Guo, Y.; Zhang, H.; Cheng, J.; Zhou, H. Inulin Improves the Egg Production Performance and Affects the Cecum Microbiota of Laying Hens. Int. J. Biol. Macromol. 2020, 155, 1599–1609. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Sun, X.; Wan, X.; Sun, G.; Jiang, R.; Li, W.; Tian, Y.; Liu, X.; Kang, X. Characteristics of the Fecal Microbiota of High- and Low-Yield Hens and Effects of Fecal Microbiota Transplantation on Egg Production Performance. Res. Vet. Sci. 2020, 129, 164–173. [Google Scholar] [CrossRef]
- Zhan, H.Q.; Dong, X.Y.; Li, L.L.; Zheng, Y.X.; Gong, Y.J.; Zou, X.T. Effects of Dietary Supplementation with Clostridium Butyricum on Laying Performance, Egg Quality, Serum Parameters, and Cecal Microflora of Laying Hens in the Late Phase of Production. Poult. Sci. 2019, 98, 896–903. [Google Scholar] [CrossRef]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary Oregano Essential Oil Supplementation Improves Intestinal Functions and Alters Gut Microbiota in Late-Phase Laying Hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef]
- Dai, D.; Wu, S.; Zhang, H.; Qi, G.; Wang, J. Dynamic Alterations in Early Intestinal Development, Microbiota and Metabolome Induced by in Ovo Feeding of L-Arginine in a Layer Chick Model. J. Anim. Sci. Biotechnol. 2020, 11, 19. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhang, H.; Wu, S.; Qi, G. Effects of Clostridium Butyricum on Production Performance and Intestinal Absorption Function of Laying Hens in the Late Phase of Production. Anim. Feed Sci. Technol. 2020, 264, 114476. [Google Scholar] [CrossRef]
- Miao, L.; Gong, Y.; Li, H.; Xie, C.; Xu, Q.; Dong, X.; Elwan, H.A.M.; Zou, X. Alterations in Cecal Microbiota and Intestinal Barrier Function of Laying Hens Fed on Fluoride Supplemented Diets. Ecotoxicol. Environ. Saf. 2020, 193, 110372. [Google Scholar] [CrossRef]
- Dai, D.; Qi, G.; Wang, J.; Zhang, H.; Qiu, K.; Wu, S. Intestinal Microbiota of Layer Hens and Its Association with Egg Quality and Safety. Poult. Sci. 2022, 101, 102008. [Google Scholar] [CrossRef]
- Wang, W.-W.; Wang, J.; Zhang, H.-J.; Wu, S.-G.; Qi, G.-H. Supplemental Clostridium Butyricum Modulates Lipid Metabolism Through Shaping Gut Microbiota and Bile Acid Profile of Aged Laying Hens. Front. Microbiol. 2020, 11, 600. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Li, X.; Yang, X.; Long, F.; Yang, X. Effects of Encapsulated Essential Oils and Organic Acids on Laying Performance, Egg Quality, Intestinal Morphology, Barrier Function, and Microflora Count of Hens during the Early Laying Period. Poult. Sci. 2019, 98, 6751–6760. [Google Scholar] [CrossRef]
- Rebollada-Merino, A.; Bárcena, C.; Ugarte-Ruiz, M.; Porras, N.; Mayoral-Alegre, F.J.; Tomé-Sánchez, I.; Domínguez, L.; Rodríguez-Bertos, A. Effects on Intestinal Mucosal Morphology, Productive Parameters and Microbiota Composition after Supplementation with Fermented Defatted Alperujo (FDA) in Laying Hens. Antibiotics 2019, 8, 215. [Google Scholar] [CrossRef]
- Herrero-Encinas, J.; Blanch, M.; Pastor, J.J.; Menoyo, D. Diet Supplementation with a Bioactive Pomace Extract from Olea Europaea Partially Mitigates Negative Effects on Gut Health Arising from a Short-Term Fasting Period in Broiler Chickens. Animal 2020, 10, 349. [Google Scholar] [CrossRef]
- Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition (Text with EEA Relevance). Off. J. L 2003, 268, 29–43.
- Alternatives to Antibiotics for Farm Animals. CABI Reviews. Available online: https://www.cabidigitallibrary.or (accessed on 31 January 2024).
- Ferlisi, F.; Tang, J.; Cappelli, K.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Oil Co-Products Rich in Polyphenols: A Novel Nutraceutical Approach in Monogastric Animal Nutrition. Front. Vet. Sci. 2023, 10, 1272274. [Google Scholar] [CrossRef]
- de Oliveira, C.O.; Roll, A.A.P.; Medeiros Gonçalves, F.M.; Lopes, D.C.N.; Xavier, E.G. Olive Pomace for the Feeding of Commercial Poultry: Effects on Performance, Meat and Eggs Quality, Haematological Parameters, Microbiota and Immunity. Worlds Poult. Sci. J. 2021, 77, 363–376. [Google Scholar] [CrossRef]
- Dedousi, A.; Kritsa, M.-Z.; Đukić Stojčić, M.; Sfetsas, T.; Sentas, A.; Sossidou, E. Production Performance, Egg Quality Characteristics, Fatty Acid Profile and Health Lipid Indices of Produced Eggs, Blood Biochemical Parameters and Welfare Indicators of Laying Hens Fed Dried Olive Pulp. Sustainability 2022, 14, 3157. [Google Scholar] [CrossRef]
- Dedousi, A.; Kotzamanidis, C.; Kritsa, M.-Z.; Tsoureki, A.; Andreadelli, A.; Patsios, S.I.; Sossidou, E. Growth Performance, Gut Health, Welfare and Qualitative Behavior Characteristics of Broilers Fed Diets Supplemented with Dried Common (Olea Europaea) Olive Pulp. Sustainability 2023, 15, 501. [Google Scholar] [CrossRef]
- Dedousi, A.; Kritsa, M.-Z.; Sossidou, E.N. Thermal Comfort, Growth Performance and Welfare of Olive Pulp Fed Broilers during Hot Season. Sustainability 2023, 15, 10932. [Google Scholar] [CrossRef]
- Habib, H.G.; Al-Zamili, I.F.; Al-Gharawi, J.K. Effect of Different Levels of Olive Pomace on Some Productive Traits of ISA Brown Laying Hens. IOP Conf. Ser. Earth Environ. Sci. 2023, 1225, 012039. [Google Scholar] [CrossRef]
- Sateri, S.; Seidavi, A.; Bouyeh, M.; Neumann, P.; Kutzler, M.; Laudadio, V.; Loperfido, F.; Tufarelli, V. Effect of Olive Meal and Supplemental Enzymes on Performance Traits, Blood Biochemistry, Humoral Immunity Response and Caecal Microbiota of Broilers. South Afr. J. Anim. Sci. 2017, 47, 804–812. [Google Scholar] [CrossRef]
- Herrero-Encinas, J.; Blanch, M.; Pastor, J.J.; Mereu, A.; Ipharraguerre, I.R.; Menoyo, D. Effects of a Bioactive Olive Pomace Extract from Olea Europaea on Growth Performance, Gut Function, and Intestinal Microbiota in Broiler Chickens. Poult. Sci. 2020, 99, 2–10. [Google Scholar] [CrossRef]
- Debbou-Iouknane, N.; Nerín, C.; Amrane, M.; Ghemghar, M.; Madani, K.; Ayad, A. In Vitro Anticoccidial Activity of Olive Pulp (Olea Europaea L. Var. Chemlal) Extract Against Eimeria Oocysts in Broiler Chickens. Acta Parasitol. 2019, 64, 887–897. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial Diversity and Community Composition of Caecal Microbiota in Commercial and Indigenous Indian Chickens Determined Using 16s rDNA Amplicon Sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Directive-1999/74-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/1999/74/oj/eng (accessed on 31 January 2024).
- Available online: https://www.joiceandhill.co.uk/documents/1362/ISA_Brown_Management_Guide_071021.pdf (accessed on 1 February 2024).
- Nielsen, S.S. (Ed.) Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Food Science Texts Series; Springer: Boston, MA, USA, 2010; pp. 47–53. ISBN 978-1-4419-1462-0. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Rincon, N.; Wood, D.E.; Breitwieser, F.P.; Pockrandt, C.; Langmead, B.; Salzberg, S.L.; Steinegger, M. Metagenome Analysis Using the Kraken Software Suite. Nat. Protoc. 2022, 17, 2815–2839. [Google Scholar] [CrossRef] [PubMed]
- CRAN: Package Vegan. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 26 August 2024).
- Lin, H.; Peddada, S.D. Multigroup Analysis of Compositions of Microbiomes with Covariate Adjustments and Repeated Measures. Nat. Methods 2024, 21, 83–91. [Google Scholar] [CrossRef]
- Mia. Available online: http://bioconductor.org/packages/mia/ (accessed on 26 August 2024).
- Create Elegant Data Visualisations Using the Grammar of Graphics. Available online: https://ggplot2.tidyverse.org/ (accessed on 26 August 2024).
- Download Previous Versions of JASP. Available online: https://jasp-stats.org/previous-versions/ (accessed on 1 February 2024).
- El-Galil, A. Physiological Responses and Productive Performance Of Laying Hens Fed Olive Cake under South Sinai Conditions. Egypt. Poult. Sci. J. 2018, 37, 293–304. [Google Scholar]
- Al-Harthi, M. The Effect of Different Dietary Contents of Olive Cake with or without Saccharomyces Cerevisiae on Egg Production and Quality, Inner Organs and Blood Constituents of Commercial Layers. Eur. Poult. Sci. EPS 2015, 79, 1–14. [Google Scholar] [CrossRef]
- Al-Harthi, M.A.; Attia, Y.A. Effect of Citric Acid on the Utilization of Olive Cake Diets for Laying Hens. Ital. J. Anim. Sci. 2015, 14, 3966. [Google Scholar] [CrossRef]
- Rezar, V.; Levart, A.; Salobir, J. The Effect of Olive by Products and Their Extracts on Antioxidative Status of Laying Hens and Oxidative Stability of Eggs Enriched with N-3 Fatty Acids. Poljoprivreda 2015, 21, 216–219. [Google Scholar] [CrossRef]
- Zangeneh, S.; Torki, M. Effects of B-Mannanase Supplementing of Olive Pulp-Included Diet on Performance of Laying Hens, Egg Quality Characteristics, Humoral and Cellular Immune Response and Blood Parameters. Glob. Vet. 2011, 7, 391–398. [Google Scholar]
- Mohebbifar, A.; Afsari, M.; Torki, M. Egg Quality Characteristics and Productive Performance of Laying Hens Fed Olive Pulp Included Diets Supplemented with Enzyme. Glob. Vet. 2011, 6, 409–416. [Google Scholar]
- Afsari, M.; Mohebbifar, A.; Torki, M. Effects of Dietary Inclusion of Olive Pulp Supplemented with Probiotics on Productive Performance, Egg Quality and Blood Parameters of Laying Hens. Annu. Res. Rev. Biol. 2014, 4, 198–211. [Google Scholar] [CrossRef]
- Ghasemi, R.; Torki, M.; Ghasemi, H.A.; Zarei, M. Single or Combined Effects of Date Pits and Olive Pulps on Productive Traits, Egg Quality, Serum Lipids and Leucocytes Profiles of Laying Hens. J. Appl. Anim. Res. 2014, 42, 103–109. [Google Scholar] [CrossRef]
- Zarei, M.; Ehsani, M.; Torki, M. Productive Performance of Laying Hens Fed Wheat-Based Diets Included Olive Pulp with or without a Commercial Enzyme Product. Afr. J. Biotechnol. 2011, 10, 4303–4312. [Google Scholar] [CrossRef]
- Dedousi, A.; Đukić Stojčić, M.; Sossidou, E. Effects of Housing Systems on Keel Bone Damage and Egg Quality of Laying Hens. Vet. Res. Forum 2020, 11, 299–304. [Google Scholar] [CrossRef]
- Đukić Stojčić, M.; Peric, L.; Spiridonovic, S. Quality of Table Eggs from Different Production Systems. Contemp. Agric. 2023, 72, 38–42. [Google Scholar] [CrossRef]
- Hamilton, R.M.G.; Bryden, W.L. Relationship between Egg Shell Breakage and Laying Hen Housing Systems—An Overview. Worlds Poult. Sci. J. 2021, 77, 249–266. [Google Scholar] [CrossRef]
- Habeeb, A.A.M.; Gad, A.E.; EL-Tarabany, A.A.; Mustafa, M.M.; Atta, M.A.A. Using of Olive Oil By-Products in Farm Animals Feeding. Cellulose 2017, 3, 57–68. [Google Scholar]
- Stefanello, C.; Santos, T.C.; Murakami, A.E.; Martins, E.N.; Carneiro, T.C. Productive Performance, Eggshell Quality, and Eggshell Ultrastructure of Laying Hens Fed Diets Supplemented with Organic Trace Minerals. Poult. Sci. 2014, 93, 104–113. [Google Scholar] [CrossRef]
- Hajjarmanesh, M.; Zaghari, M.; Hajati, H.; Ahmad, A.H. Effects of Zinc, Manganese, and Taurine on Egg Shell Microstructure in Commercial Laying Hens after Peak Production. Biol. Trace Elem. Res. 2023, 201, 2982–2990. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Kim, I.H. Effects of Dietary Olive Oil on Egg Quality, Serum Cholesterol Characteristics, and Yolk Fatty Acid Concentrations in Laying Hens. J. Appl. Anim. Res. 2014, 42, 233–237. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Sabic, E.M.; Abu-Taleb, A.M. Effect of Inclusion Irradiated Olive Pulp in Laying Quail Diets on Biological Performance. J. Radiat. Res. Appl. Sci. 2018, 11. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Salem, H.M.; Khafaga, A.F.; Soliman, S.M.; El-Saadony, M.T. Impacts of Polyphenols on Laying Hens’ Productivity and Egg Quality: A Review. J. Anim. Physiol. Anim. Nutr. 2023, 107, 928–947. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Q.; Shao, D.; Sheng, Z.W.; Wang, Q.; Shi, S.R. A Mixture of Daidzein and Chinese Herbs Increases Egg Production and Eggshell Strength as Well as Blood Plasma Ca, P, Antioxidative Enzymes, and Luteinizing Hormone Levels in Post-Peak, Brown Laying Hens. Poult. Sci. 2019, 98, 3298–3303. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Bai, H.; Wang, C.; Wang, F.; Yuan, Q. Curcumin Improves the Egg Quality, Antioxidant Activity, and Intestinal Microbiota of Quails during the Late Laying Period. Poult. Sci. 2024, 103, 103233. [Google Scholar] [CrossRef] [PubMed]
- Sayehban, P.; Seidavi, A.; Dadashbeiki, M.; Ghorbani, A.; Araújo, W.; Albino, L. Effects of Different Levels of Two Types of Olive Pulp with or without Exogenous Enzyme Supple-Mentation on Broiler Performance and Economic Parameters. Rev. Bras. Ciênc. Avícola 2016, 18, 489–500. [Google Scholar] [CrossRef]
- Al-Harthi, M.A. The Effect of Olive Cake, with or without Enzymes Supplementation, on Growth Performance, Carcass Characteristics, Lymphoid Organs and Lipid Metabolism of Broiler Chickens. Braz. J. Poult. Sci. 2017, 19, 83–90. [Google Scholar] [CrossRef]
- Kheravii, S.K.; Morgan, N.K.; Swick, R.A.; Choct, M.; Wu, S.-B. Roles of Dietary Fibre and Ingredient Particle Size in Broiler Nutrition. Worlds Poult. Sci. J. 2018, 74, 301–316. [Google Scholar] [CrossRef]
- Desbruslais, A.; Wealleans, A.; Gonzalez-Sanchez, D.; di Benedetto, M. Dietary Fibre in Laying Hens: A Review of Effects on Performance, Gut Health and Feather Pecking. Worlds Poult. Sci. J. 2021, 77, 797–823. [Google Scholar] [CrossRef]
- Sozcu, A.; Ipek, A. The Effects of Lignocellulose Supplementation on Laying Performance, Egg Quality Parameters, Aerobic Bacterial Load of Eggshell, Serum Biochemical Parameters, and Jejunal Histomorphological Traits of Laying Hens. Poult. Sci. 2020, 99, 3179–3187. [Google Scholar] [CrossRef]
- Röhe, I.; Urban, J.; Dijkslag, A.; Te Paske, J.; Zentek, J. Impact of an Energy- and Nutrient-Reduced Diet Containing 10% Lignocellulose on Animal Performance, Body Composition and Egg Quality of Dual Purpose Laying Hens. Arch. Anim. Nutr. 2019, 73, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Koçer, B.; Bozkurt, M.; Ege, G.; Tüzün, A.E. Effects of Sunflower Meal Supplementation in the Diet on Productive Performance, Egg Quality and Gastrointestinal Tract Traits of Laying Hens. Br. Poult. Sci. 2021, 62, 101–109. [Google Scholar] [CrossRef]
- Kraus, A.; Zita, L. The Effect of Age and Genotype on Quality of Eggs in Brown Egg-Laying Hybrids. Acta Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 407–414. [Google Scholar] [CrossRef]
- Peric, L.; Đukić Stojčić, M.; Bjedov, S. The Effect of Storage and Age of Hens on the Quality of Table Eggs. Adv. Res. Life Sci. 2017, 1, 64–67. [Google Scholar] [CrossRef]
- Benavides-Reyes, C.; Folegatti, E.; Dominguez-Gasca, N.; Litta, G.; Sanchez-Rodriguez, E.; Rodriguez-Navarro, A.B.; Umar Faruk, M. Research Note: Changes in Eggshell Quality and Microstructure Related to Hen Age during a Production Cycle. Poult. Sci. 2021, 100, 101287. [Google Scholar] [CrossRef] [PubMed]
- Wistedt, A.; Ridderstråle, Y.; Wall, H.; Holm, L. Age-Related Changes in the Shell Gland and Duodenum in Relation to Shell Quality and Bone Strength in Commercial Laying Hen Hybrids. Acta Vet. Scand. 2019, 61, 14. [Google Scholar] [CrossRef]
- Roberts, J.; Chousalkar, K.; Khan, S. Egg Quality and Age of Laying Hens: Implications for Product Safety. Anim. Prod. Sci. 2013, 53, 1291–1297. [Google Scholar] [CrossRef]
- Tumová, E.; Ledvinka, Z. The Effect of Time of Oviposition and Age on Egg Weight, Egg Components Weight and Eggshell Quality. Arch. Geflugelkd. 2009, 73, 110–115. [Google Scholar]
- Padhi, M.; Chatterjee, R.; Haunshi, S.; Ullengala, R. Effect of Age on Egg Quality in Chicken. Indian J. Poult. Sci. 2013, 48, 122–125. [Google Scholar]
- de Andrade, P.G.C.; Mendonça, M.A.d.F.; Cruz, F.G.G.; Rufino, J.P.F.; Silva, F.M.F.; Reis, L.d.A. Effects of dietary fiber on performance and egg quality of laying hens at pre-laying and laying peak. Acta Sci. Anim. Sci. 2023, 45, e57534. [Google Scholar] [CrossRef]
- Biesiada-Drzazga, B.B.; Banaszewska, D.; Kaim-Mirowski, S.B. Analysis of Selected External and Internal Characteristics of the Eggs of Hy-Line Brown Hens in Relation to Their Age. Anim. Sci. Genet. 2022, 18, 45–56. [Google Scholar] [CrossRef]
- Shell Egg Grades and Standards Agricultural Marketing Service. Available online: https://www.ams.usda.gov/grades-standards/shell-egg-grades-and-standards (accessed on 28 August 2024).
- Nys, Y. Dietary Carotenoids and Egg Yolk Coloration—A Review. Arch. Geflugelkd. 2000, 64, 45–54. [Google Scholar]
- Zurak, D.; Slovenec, P.; Janječić, Z.; Bedeković, X.D.; Pintar, J.; Kljak, K. Overview on Recent Findings of Nutritional and Non-Nutritional Factors Affecting Egg Yolk Pigmentation. Worlds Poult. Sci. J. 2022, 78, 531–560. [Google Scholar] [CrossRef]
- Ruesga-Gutiérrez, E.; Ruvalcaba-Gómez, J.M.; Gómez-Godínez, L.J.; Villagrán, Z.; Gómez-Rodríguez, V.M.; Heredia-Nava, D.; Ramírez-Vega, H.; Arteaga-Garibay, R.I. Allium-Based Phytobiotic for Laying Hens’ Supplementation: Effects on Productivity, Egg Quality, and Fecal Microbiota. Microorganisms 2022, 10, 117. [Google Scholar] [CrossRef]
- Sousa, L.S.D.; Carvalho, T.S.M.; Nogueira, F.A.; Saldanha, M.M.; Vaz, D.P.; Bertechini, A.G.; Baião, N.C.; Lara, L.J.C. Fiber Source and Xylanase on Performance, Egg Quality, and Gastrointestinal Tract of Laying Hens. Rev. Bras. Zootec. 2019, 48, e20170286. [Google Scholar] [CrossRef]
- Han, G.P.; Kim, D.Y.; Kim, K.H.; Kim, J.H.; Kil, D.Y. Effect of Dietary Concentrations of Metabolizable Energy and Neutral Detergent Fiber on Productive Performance, Egg Quality, Fatty Liver Incidence, and Hepatic Fatty Acid Metabolism in Aged Laying Hens. Poult. Sci. 2023, 102, 102497. [Google Scholar] [CrossRef] [PubMed]
- Banaszewska, D.; Biesiada-Drzazga, B.; Ostrowski, D.; Drabik, K.; Batkowska, J. The Impact of Breeder Age on Egg Quality and Lysozyme Activity. Turk. J. Vet. Anim. Sci. 2019, 43, 583–589. [Google Scholar] [CrossRef]
- Marzec, A.; Damaziak, K.; Kowalska, H.; Riedel, J.; Michalczuk, M.; Koczywąs, E.; Cisneros, F.; Lenart, A.; Niemiec, J. Effect of Hens Age and Storage Time on Functional and Physiochemical Properties of Eggs. J. Appl. Poult. Res. 2019, 28, 290–300. [Google Scholar] [CrossRef]
- Krawczyk, J.; Lewko, L.; Sokołowicz, Z.; Koseniuk, A.; Kraus, A. Effect of Hen Genotype and Laying Time on Egg Quality and Albumen Lysozyme Content and Activity. Animals 2023, 13, 1611. [Google Scholar] [CrossRef]
- Vlčková, J.; Tůmová, E.; Míková, K.; Englmaierová, M.; Okrouhlá, M.; Chodová, D. Changes in the Quality of Eggs during Storage Depending on the Housing System and the Age of Hens. Poult. Sci. 2019, 98, 6187–6193. [Google Scholar] [CrossRef]
- Poletti, B.; Vieira, M. Shelf Life of Brown Eggs from Laying Hens of Different Ages in Organic Production System/Vida de Prateleira de Ovos Marrons de Poedeiras Com Diferentes Idades de Postura Em Sistema Orgânico de Produção. Braz. J. Anim. Environ. Res. 2021, 4, 2–15. [Google Scholar] [CrossRef]
- Dong, X.; Yin, Z.; Ma, Y.; Cao, H.; Dong, D. Effects of Rearing Systems on Laying Performance, Egg Quality, and Serum Biochemistry of Xianju Chickens in Summer. Poult. Sci. 2017, 96, 3896–3900. [Google Scholar] [CrossRef] [PubMed]
- Straková, E.; Všetičková, L.; Kutlvašr, M.; Suchý, P. Changes in Haematological and Biochemical Blood Parameters of Laying Hens of the ROSS 308 Parent Flock during the Hatching Period. J. Anim. Feed Sci. 2022, 32, 43–49. [Google Scholar] [CrossRef]
- Osadcha, Y.V.; Sakhatsky, M.I.; Kulibaba, R.O. Serum Clinical Biochemical Markers of Hy-Line W-36 Laying Hens under the Influence of Increased Stocking Densities in Cages of Multilevel Batteries. Regul. Mech. Biosyst. 2021, 12, 425–429. [Google Scholar] [CrossRef]
- Lumeij, J.T. Chapter 28—Avian Clinical Biochemistry. In Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 839–872. ISBN 978-0-12-370491-7. [Google Scholar]
- Neijat, M.; Gakhar, N.; Neufeld, J.; House, J.D. Performance, Egg Quality, and Blood Plasma Chemistry of Laying Hens Fed Hempseed and Hempseed Oil. Poult. Sci. 2014, 93, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, A.; Pokludová, M.; Zapletal, D.; Jelínek, P. Effects of Housing Systems on Biochemical Indicators of Blood Plasma in Laying Hens. Acta Vet. Brno 2007, 76, 339–347. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Ko, Y.-H.; Moon, Y.; Sohn, S.; Jang, I.-S. Effects of Housing Systems on Physiological and Immunological Parameters in Laying Hens. J. Anim. Sci. Technol. 2013, 55, 131–139. [Google Scholar] [CrossRef][Green Version]
- Kraus, A.; Zita, L.; Krunt, O.; Härtlová, H.; Chmelíková, E. Determination of Selected Biochemical Parameters in Blood Serum and Egg Quality of Czech and Slovak Native Hens Depending on the Housing System and Hen Age. Poult. Sci. 2021, 100, 1142–1153. [Google Scholar] [CrossRef]
- Gyenis, J.; Sütő, Z.; Romvári, R.; Horn, P. Tracking the Development of Serum Biochemical Parameters in Two Laying Hen Strains—A Comparative Study. Arch Tierz Dummerstorf 2006, 49, 593–606. [Google Scholar] [CrossRef]
- Tang, S.G.H.; Sieo, C.C.; Ramasamy, K.; Saad, W.Z.; Wong, H.K.; Ho, Y.W. Performance, Biochemical and Haematological Responses, and Relative Organ Weights of Laying Hens Fed Diets Supplemented with Prebiotic, Probiotic and Synbiotic. BMC Vet. Res. 2017, 13, 248. [Google Scholar] [CrossRef]
- Usturoi, A.; Usturoi, M.-G.; Avarvarei, B.-V.; Pânzaru, C.; Simeanu, C.; Usturoi, M.-I.; Spătaru, M.; Radu-Rusu, R.-M.; Doliş, M.-G.; Simeanu, D. Research Regarding Correlation between the Assured Health State for Laying Hens and Their Productivity. Agriculture 2023, 13, 86. [Google Scholar] [CrossRef]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of Microorganisms in the Evolution of Animals and Plants: The Hologenome Theory of Evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Kogut, M.H. Spatial and Temporal Changes in the Broiler Chicken Cecal and Fecal Microbiomes and Correlations of Bacterial Taxa with Cytokine Gene Expression. Front. Vet. Sci. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Videnska, P.; Sedlar, K.; Lukac, M.; Faldynova, M.; Gerzova, L.; Cejkova, D.; Sisak, F.; Rychlik, I. Succession and Replacement of Bacterial Populations in the Caecum of Egg Laying Hens over Their Whole Life. PLoS ONE 2014, 9, e115142. [Google Scholar] [CrossRef]
- Jurburg, S.D.; Brouwer, M.S.M.; Ceccarelli, D.; van der Goot, J.; Jansman, A.J.M.; Bossers, A. Patterns of Community Assembly in the Developing Chicken Microbiome Reveal Rapid Primary Succession. Microbiologyopen 2019, 8, e00821. [Google Scholar] [CrossRef] [PubMed]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A Relevant Minority for the Maintenance of Gut Homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the Evolutionary History of an Ancient Phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Bernstein, S.; Huffman, M.A.; Xia, D.-P.; Gu, Z.; Chen, R.; Sheeran, L.K.; Wagner, R.S.; Li, J. Marked Variation between Winter and Spring Gut Microbiota in Free-Ranging Tibetan Macaques (Macaca Thibetana). Sci. Rep. 2016, 6, 26035. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important Metabolic Pathways and Biological Processes Expressed by Chicken Cecal Microbiota. Appl. Environ. Microbiol. 2016, 82, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zheng, L.; Yan, X.; Gong, L.; Yang, Y.; Qi, Q.; Zhang, X.; Zhang, H. Effects of Dietary Essential Oils Supplementation on Egg Quality, Biochemical Parameters, and Gut Microbiota of Late-Laying Hens. Animals 2022, 12, 2561. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Jahanian, R.; Golshadi, M. Effect of Dietary Supplementation of Butyric Acid Glycerides on Performance, Immunological Responses, Ileal Microflora, and Nutrient Digestibility in Laying Hens Fed Different Basal Diets. Livest. Sci. 2015, 178, 228–236. [Google Scholar] [CrossRef]
- Soltan, M. Effect of Dietary Organic Acid Supplementation on Egg Production, Egg Quality and Some Blood Serum Parameters in Laying Hens. Int. J. Poult. Sci. 2008, 7, 613–621. [Google Scholar] [CrossRef]
- Casselbrant, A.; Fändriks, L.; Wallenius, V. Glycocholic Acid and Butyrate Synergistically Increase Vitamin D-Induced Calcium Uptake in Caco-2 Intestinal Epithelial Cell Monolayers. Bone Rep. 2020, 13, 100294. [Google Scholar] [CrossRef]

| Ingredients (%) | CON | OP4 | OP6 |
|---|---|---|---|
| Maize | 56.6 | 59.0 | 57.0 |
| Soya meal—47 | 25.5 | 24.8 | 24.8 |
| Wheat bran | 5.0 | 0 | 0 |
| Olive pulp | 0 | 4.0 | 6.0 |
| Marble coarse | 3.5 | 3.5 | 3.5 |
| Marble powder | 5.5 | 5.5 | 5.5 |
| Vitamin and mineral premix 1 | 2.5 | 2.5 | 2.5 |
| Soya oil | 1.2 | 0.5 | 0.5 |
| Salt | 0.1 | 0.1 | 0.1 |
| Sodium bicarbonate | 0.1 | 0.1 | 0.1 |
| Calculated analysis | |||
| Lysine (%) | 0.82 | 0.82 | 0.82 |
| Methionine + Cystine (%) | 0.65 | 0.65 | 0.65 |
| Ca (%) | 4.25 | 4.25 | 4.25 |
| Av. P 2 (%) | 0.34 | 0.34 | 0.34 |
| Parameter | CON | OP4 | OP6 |
|---|---|---|---|
| Energy (kJ/100 g) | 1333.26 ± 34.93 | 1343.33 ± 24.86 | 1329.79 ± 38.40 |
| Fat (%) | 3.72 ± 0.27 | 3.51 ± 0.31 | 3.99 ± 0.10 |
| SFA (%) | 0.69 ± 0.16 | 0.60 ± 0.11 | 0.64 ± 0.01 |
| MUFA (%) | 1.25 ± 0.01 | 1.21 ± 0.13 | 1.30 ± 0.13 |
| PUFA (%) | 1.78 ± 0.16 | 1.70 ± 0.15 | 2.05 ± 0.19 |
| Proteins (%) | 17.50 ± 0.47 | 17.26 ± 0.52 | 17.29 ± 0.48 |
| Carbohydrates (%) | 51.74 ± 1.73 | 54.61 ±1.82 | 53.73 ± 1.24 |
| Crude Fiber (%) | 2.54 ± 1.16 | 3.03 ± 1.74 | 3.29 ± 1.49 |
| Moisture (%) | 9.54 ± 0.41 | 9.36 ± 1.05 | 9.49 ± 0.45 |
| Ash (%) | 14.96 ± 0.95 | 12.23 ± 1.34 | 12.21 ± 1.72 |
| Total polyphenols (ppm) | 95.40 ± 23.80 | 123.06 ± 37.66 | 137.24 ± 24.76 |
| Cholesterol (ppm) | <10 | <10 | <10 |
| Parameter | Olive Pulp |
|---|---|
| Energy (kJ/100 g) | 1464.3 ± 22.01 |
| Proteins (%) | 8.5 ± 0.78 |
| Carbohydrates (%) | 40.2 ± 2.73 |
| Crude Fiber (%) | 29.3 ± 4.23 |
| Moisture (%) | 4.3 ± 0.23 |
| Ash (%) | 6.9 ± 1.97 |
| Fat (%) | 10.9 ± 0.56 |
| Saturated Fatty Acids—SFA (%) | 1.7 ± 0.17 |
| Monounsaturated Fatty Acids—MUFA (%) | 7.9 ± 0.45 |
| Polyunsaturated Fatty Acids—PUFA (%) | 1.3 ± 0.05 |
| Total polyphenols (ppm) | 573.70 ± 289.31 |
| Cholesterol (ppm) | <10 |
| Eleuropein (ppm) | 20.7 ± 1.54 |
| Hydroxytyrosol (ppm) | <3 |
| Fatty Acids (g/100 g Fat) | |
|---|---|
| Lauric (dodecanoic) acid (C12:0) | 0.03 ± 0.02 |
| Myristic acid (C14:0) | 0.05 ± 0.01 |
| Palmitic acid (C16:0) | 11.23 ± 0.56 |
| Palmitoleic acid (C16:1) | 0.57 ± 0.13 |
| Margaric acid (C17:0) | 0.12 ± 0.05 |
| Cis-10-Heptadecenoic acid (C17:1) | 0.19 ± 0.10 |
| Stearic acid (C18: 0) | 2.87 ± 0.05 |
| Oleic acid (C18:1) | 71.45 ± 0.44 |
| α-Linoleic acid (C18:2) | 10.47 ± 1.12 |
| Linolenic acid(C18:3) | 1.24 ± 0.12 |
| Arachidic acid (C20:0) | 0.56 ± 0.04 |
| Arachidonic acid (C 20:4 ω6) | 0.25 ± 0.14 |
| Behenic acid (C22:0) | 0.25 ± 0.05 |
| Tricosanoic acid (C23:0) | 0.15 ± 0.13 |
| SFA | 15.3 ± 0.78 |
| MUFA | 72.6 ± 0.39 |
| PUFA | 12.1 ± 1.13 |
| Items | CON | OP4 | OP6 |
|---|---|---|---|
| Final BW (Kg) | 2.02 ± 0.02 | 2.05 ± 0.02 | 1.99 ± 0.02 |
| HDEP (%) | 94.31 ± 0.62 | 93.63 ± 0.56 | 94.30 ± 0.54 |
| Egg weight (g) | 64.25 ± 0.38 | 63.16 ± 0.40 | 63.80 ± 0.40 |
| ADFI (g/h/d) | 207.60 ± 5.17 | 210.61 ± 5.17 | 210.81 ± 5.01 |
| Egg mass | 60.83 ± 0.62 | 59.15 ± 0.54 | 60.18 ± 0.58 |
| FCR | 3.42 ± 0.08 | 3.57 ± 0.09 | 3.50 ± 0.07 |
| Eggs with broken shell % | 0.53 ± 0.08 a | 0.35 ± 0.05 b | 0.45 ± 0.07 ab |
| Eggs with dirty eggshells % | 4.80 ± 0.54 | 5.71 ± 0.56 | 6.36 ± 0.72 |
| Parameter | Group | WK39 | WK59 | Group Mean | P | ||
|---|---|---|---|---|---|---|---|
| Group | Age | Group × Age | |||||
| Egg weight | CON | 63.25 ± 1.18 | 66.03 ± 1.18 | 64.64 ± 0.83 | 0.152 | 0.045 | 0.774 |
| OP4 | 61.42 ± 1.18 | 63.25 ± 1.18 | 62.34 ± 0.83 | ||||
| OP6 | 63.13 ± 1.18 | 64.22 ± 1.18 | 63.68 ± 0.83 | ||||
| Age mean | 62.60 ± 0.68 A | 64.50 ± 0.68 B | |||||
| Egg width | CON | 44.24 ± 0.33 | 45.25 ± 0.33 a | 44.74 ± 0.23 a | <0.001 | 0.721 | <0.001 |
| OP4 | 44.21 ± 0.33 A | 42.53 ± 0.33 bB | 43.37 ± 0.23 b | ||||
| OP6 | 44.27 ± 0.33 | 44.65 ± 0.33 a | 44.46 ± 0.23 a | ||||
| Age mean | 44.24 ± 0.19 | 44.14 ± 0.19 | |||||
| Egg length | CON | 56.69 ± 0.49 | 56.88 ± 0.49 | 56.78 ± 0.34 | 0.078 | 0.348 | 0.826 |
| OP4 | 55.39 ± 0.49 | 56.11 ± 0.49 | 55.75 ± 0.34 | ||||
| OP6 | 56.51 ± 0.49 | 56.73 ± 0.49 | 56.62 ± 0.34 | ||||
| Age mean | 56.20 ± 0.28 | 56.57 ± 0.28 | |||||
| Shape index (%) | CON | 78.09 ± 0.76 | 79.65 ± 0.76 a | 78.87 ± 0.54 | 0.390 | 0.242 | <0.001 |
| OP4 | 79.90 ± 0.76 A | 75.82 ± 0.76 bB | 77.86 ± 0.54 | ||||
| OP6 | 78.42 ± 0.76 | 78.76 ± 0.76 ab | 78.59 ± 0.54 | ||||
| Age mean | 78.80 ± 0.44 | 78.08 ± 0.44 | |||||
| SBS (N) | CON | 52.68 ± 2.27 | 45.69 ± 2.27 | 49.18 ± 1.61 | 0.849 | 0.017 | 0.415 |
| OP4 | 51.54 ± 2.27 | 46.13 ± 2.27 | 48.84 ± 1.61 | ||||
| OP6 | 50.67 ± 2.27 | 49.53 ± 2.27 | 50.10 ± 1.61 | ||||
| Age mean | 51.63 ± 1.31 A | 47.12 ± 1.31 B | |||||
| Deformation (mm) | CON | 0.95 ± 0.03 | 0.83 ± 0.03 | 0.89 ± 0.02 | 0.492 | <0.001 | 0.400 |
| OP4 | 0.91 ± 0.03 | 0.79 ± 0.03 | 0.85 ± 0.02 | ||||
| OP6 | 0.88 ± 0.03 | 0.84 ± 0.03 | 0.86 ± 0.02 | ||||
| Age mean | 0.91 ± 0.02 A | 0.82 ± 0.02 B | |||||
| Albumen weight (gr) | CON | 35.49 ± 0.89 | 36.47 ± 0.89 | 35.98 ± 0.63 | 0.212 | 0.873 | 0.620 |
| OP4 | 34.32 ± 0.89 | 34.46 ± 0.89 | 34.39 ± 0.63 | ||||
| OP6 | 35.58 ± 0.89 | 34.81 ± 0.89 | 35.20 ± 0.63 | ||||
| Age mean | 35.13 ± 0.51 | 35.25 ± 0.51 | |||||
| Albumen ratio (%) | CON | 56.05 ± 0.66 | 55.23 ± 0.66 | 55.64 ± 0.47 | 0.700 | 0.007 | 0.631 |
| OP4 | 55.90 ± 0.66 | 54.33 ± 0.66 | 55.12 ± 0.47 | ||||
| OP6 | 56.24 ± 0.66 | 54.15 ± 0.66 | 55.20 ± 0.47 | ||||
| Age mean | 56.06 ± 0.38 A | 54.57 ± 0.38 B | |||||
| Yolk weight (gr) | CON | 18.66 ± 0.41 a | 17.60 ± 0.41 | 18.13 ± 0.29 a | <0.001 | 0.865 | 0.061 |
| OP4 | 15.58 ± 0.41 b | 16.32 ± 0.41 | 15.95 ± 0.29 b | ||||
| OP6 | 15.84 ± 0.41 b | 16.33 ± 0.41 | 16.08 ± 0.29 b | ||||
| Age mean | 16.69 ± 0.23 | 16.75 ± 0.23 | |||||
| Yolk ratio (%) | CON | 29.58 ± 0.54 aA | 26.62 ± 0.54 B | 28.10 ± 0.38 a | <0.001 | 0.123 | 0.002 |
| OP4 | 25.32 ± 0.54 b | 25.88 ± 0.54 | 25.60 ± 0.38 b | ||||
| OP6 | 25.14 ± 0.54 b | 25.48 ± 0.54 | 25.31 ± 0.38 b | ||||
| Age mean | 26.68 ± 0.31 | 25.99 ± 0.31 | |||||
| Shell weight (gr) | CON | 7.65 ± 0.18 | 8.31 ± 0.18 | 7.98 ± 0.12 | 0.079 | 0.007 | 0.433 |
| OP4 | 7.57 ± 0.18 | 7.84 ± 0.18 | 7.70 ± 0.12 | ||||
| OP6 | 7.47 ± 0.18 | 7.73 ± 0.18 | 7.60 ± 0.12 | ||||
| Age mean | 7.56 ± 0.10 A | 7.96 ± 0.10 B | |||||
| Shell ratio (%) | CON | 12.10 ± 0.22 | 12.59 ± 0.22 | 12.34 ± 0.16 | 0.100 | 0.141 | 0.634 |
| OP4 | 12.33 ± 0.22 | 12.42 ± 0.22 | 12.38 ± 0.15 | ||||
| OP6 | 11.85 ± 0.22 | 12.05 ± 0.22 | 11.95 ± 0.16 | ||||
| Age mean | 12.09 ± 0.13 | 12.36 ± 0.13 | |||||
| Albumen height (mm) | CON | 8.31 ± 0.26 | 8.29 ± 0.26 | 8.30 ± 0.19 | 0.060 | 0.222 | 0.387 |
| OP4 | 7.42 ± 0.26 | 8.10 ± 0.26 | 7.76 ± 0.19 | ||||
| OP6 | 7.68 ± 0.26 | 7.82 ± 0.26 | 7.75 ± 0.19 | ||||
| Age mean | 7.80 ± 0.15 | 8.07 ± 0.15 | |||||
| Haugh unit | CON | 90.05 ± 1.44 | 89.53 ± 1.44 | 89.79 ± 1.02 | 0.090 | 0.263 | 0.318 |
| OP4 | 85.36 ± 1.44 | 89.10 ± 1.44 | 87.23 ± 1.02 | ||||
| OP6 | 86.49 ± 1.44 | 87.23 ± 1.44 | 86.86 ± 1.02 | ||||
| Age mean | 87.30 ± 0.83 | 88.62 ± 0.83 | |||||
| Shell thickness (mm) | CON | 0.69 ± 0.02 A | 0.44 ± 0.02 aB | 0.56 ± 0.01 a | <0.001 | <0.001 | <0.001 |
| OP4 | 0.68 ± 0.02 | 0.66 ± 0.02 b | 0.67 ± 0.01 b | ||||
| OP6 | 0.68 ± 0.02 | 0.63 ± 0.02 b | 0.66 ± 0.01 b | ||||
| Age mean | 0.68 ± 0.01 A | 0.58 ± 0.01 B | |||||
| Yolk color | CON | 6.80 ± 0.20 aA | 8.60 ± 0.20 B | 7.70 ± 0.14 a | <0.001 | <0.001 | <0.001 |
| OP4 | 6.93 ± 0.20 aA | 8.93 ± 0.20 B | 7.93 ± 0.14 a | ||||
| OP6 | 9.20 ± 0.20 bA | 8.33 ± 0.20 B | 8.77 ± 0.14 b | ||||
| Age mean | 7.64 ± 0.12 A | 8.62 ± 0.12 B | |||||
| Yolk height (mm) | CON | 18.66 ± 0.27 | 18.72 ± 0.27 | 18.69 ± 0.20 | 0.063 | 0.163 | 0.061 |
| OP4 | 18.06 ± 0.27 | 18.16 ± 0.27 | 18.11 ± 0.20 | ||||
| OP6 | 18.70 ± 0.27 | 17.63 ± 0.20 | 18.16 ± 0.20 | ||||
| Age mean | 18.48 ± 0.16 | 18.17 ± 0.16 | |||||
| Yolk width (mm) | CON | 41.63 ± 0.47 | 42.84 ± 0.47 | 42.23 ± 0.33 | 0.224 | <0.001 | 0.405 |
| OP4 | 40.33 ± 0.47 A | 42.53 ± 0.47 B | 41.43 ± 0.33 | ||||
| OP6 | 41.43 ± 0.47 | 42.45 ± 0.47 | 41.94 ± 0.33 | ||||
| Age mean | 41.13 ± 0.27 A | 42.61 ± 0.27 B | |||||
| Yolk index | CON | 0.45 ± 0.01 | 0.44 ± 0.01 | 0.44 ± 0.01 | 0.538 | <0.001 | 0.291 |
| OP4 | 0.45 ± 0.01 | 0.43 ± 0.01 | 0.44 ± 0.01 | ||||
| OP6 | 0.45 ± 0.01 A | 0.42 ± 0.01 B | 0.43 ± 0.01 | ||||
| Age mean | 0.45 ± 0.01 A | 0.43 ± 0.01 B | |||||
| Albumen pH | CON | 8.74 ± 0.07 A | 8.30 ± 0.07 Βa | 8.52 ± 0.05 a | <0.001 | <0.001 | <0.001 |
| OP4 | 8.90 ± 0.07 A | 8.39 ± 0.07 Βa | 8.64 ± 0.05 a | ||||
| OP6 | 8.80 ± 0.07 | 8.84 ± 0.07 b | 8.82 ± 0.05 b | ||||
| Age mean | 8.81 ± 0.04 A | 8.51 ± 0.04 B | |||||
| Yolk pH | CON | 6.04 ± 0.09 | 5.82 ± 0.09 | 5.93 ± 0.06 | 0.248 | <0.001 | 0.445 |
| OP4 | 6.28 ± 0.09 A | 5.87 ± 0.09 B | 6.08 ± 0.06 | ||||
| OP6 | 6.11 ± 0.09 | 5.90 ± 0.09 | 6.01 ± 0.06 | ||||
| Age mean | 6.15 ± 0.05 A | 5.86 ± 0.06 B | |||||
| Parameter | Group | WK39 | WK59 | Group Mean | P | ||
|---|---|---|---|---|---|---|---|
| Group | Age | Group × Age | |||||
| Cholesterol (mg/dL) | CON | 124.07 ± 8.56 | 117.20 ± 8.56 | 120.63 ± 6.05 | 0.895 | 0.065 | 0.205 |
| OP4 | 133.81 ± 8.29 | 103.47 ± 8.56 | 118.64 ± 5.96 | ||||
| OP6 | 123.53 ± 8.56 | 121.73 ± 8.56 | 122.63 ± 6.05 | ||||
| Age mean | 127.14 ± 4.89 | 114.13 ± 4.94 | |||||
| Triglycerides (mg/dL) | CON | 1778.93 ± 193.39 | 1762.33 ± 193.39 | 1770.63 ± 136.75 | 0.833 | 0.327 | 0.533 |
| OP4 | 1867.19 ± 187.25 | 1462.60 ± 193.39 | 1664.89 ± 134.60 | ||||
| OP6 | 1781.87 ± 193.39 | 1735.86 ± 200.18 | 1758.86 ± 139.17 | ||||
| Age mean | 1809.33 ± 110.49 | 1653.60 ± 112.98 | |||||
| AST (IU/L) | CON | 131.00 ± 3.81 A | 114.40 ± 3.81 B | 122.70 ± 2.69 | 0.095 | <0.001 | 0.086 |
| OP4 | 133.44 ± 3.69 | 125.93 ± 3.81 | 129.69 ± 2.65 | ||||
| OP6 | 142.40 ± 3.81 A | 117.93 ± 3.81 B | 130.17 ± 2.69 | ||||
| Age mean | 135.61 ± 2.18 A | 119.42 ± 2.20 B | |||||
| G-GT (IU/L) | CON | 25.53 ± 2.66 aA | 36.93 ± 2.66 B | 31.23 ± 1.88 a | <0.001 | 0.781 | 0.002 |
| OP4 | 43.06 ± 2.57 b | 35.93 ± 2.66 | 39.50 ± 1.85 b | ||||
| OP6 | 42.00 ± 2.66 b | 39.53 ± 2.66 | 40.77 ± 1.88 b | ||||
| Age mean | 36.87 ± 1.52 | 37.47 ± 1.53 | |||||
| Uric acid (mg/dL) | CON | 7.88 ± 0.35 | 7.00 ± 0.36 | 7.44 ± 0.26 | 0.210 | <0.001 | 0.440 |
| OP4 | 7.66 ± 0.34 A | 6.04 ± 0.35 B | 6.85 ± 0.24 | ||||
| OP6 | 7.81 ± 0.35 A | 6.08 ± 0.35 B | 6.94 ± 0.25 | ||||
| Age mean | 7.78 ± 0.20 A | 6.37 ± 0.21 B | |||||
| BUN (mg/dL) | CON | 11.00 ± 0.83 | 11.47 ± 0.83 | 11.23 ± 0.59 | 0.749 | 0.734 | 0.564 |
| OP4 | 11.63 ± 0.81 | 10.40 ± 0.83 | 11.01 ± 0.58 | ||||
| OP6 | 11.60 ± 0.83 | 11.67 ± 0.83 | 11.63 ± 0.59 | ||||
| Age mean | 11.41 ± 0.48 | 11.18 ± 0.48 | |||||
| GLDH (U/L) | CON | 4.07 ± 0.34 | 2.67 ± 0.34 | 3.37 ± 0.24 a | 0.014 | 0.001 | 0.519 |
| OP4 | 4.75 ± 0.34 | 3.93 ± 0.35 | 4.34 ± 0.24 b | ||||
| OP6 | 3.87 ± 0.34 | 3.23 ± 0.37 | 3.55 ± 0.25 ab | ||||
| Age mean | 4.23 ± 0.20 A | 3.28 ± 0.21 B | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedousi, A.; Kotzamanidis, C.; Malousi, A.; Giantzi, V.; Sossidou, E. The Influence of Dietary Supplementation with Dried Olive Pulp on Gut Microbiota, Production Performance, Egg Quality Traits, and Health of Laying Hens. Microorganisms 2024, 12, 1916. https://doi.org/10.3390/microorganisms12091916
Dedousi A, Kotzamanidis C, Malousi A, Giantzi V, Sossidou E. The Influence of Dietary Supplementation with Dried Olive Pulp on Gut Microbiota, Production Performance, Egg Quality Traits, and Health of Laying Hens. Microorganisms. 2024; 12(9):1916. https://doi.org/10.3390/microorganisms12091916
Chicago/Turabian StyleDedousi, Anna, Charalampos Kotzamanidis, Andigoni Malousi, Virginia Giantzi, and Evangelia Sossidou. 2024. "The Influence of Dietary Supplementation with Dried Olive Pulp on Gut Microbiota, Production Performance, Egg Quality Traits, and Health of Laying Hens" Microorganisms 12, no. 9: 1916. https://doi.org/10.3390/microorganisms12091916
APA StyleDedousi, A., Kotzamanidis, C., Malousi, A., Giantzi, V., & Sossidou, E. (2024). The Influence of Dietary Supplementation with Dried Olive Pulp on Gut Microbiota, Production Performance, Egg Quality Traits, and Health of Laying Hens. Microorganisms, 12(9), 1916. https://doi.org/10.3390/microorganisms12091916





