Self-Organized Heterocyclic Amines Films on Carbon Substrates for Photovoltaic Applications
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
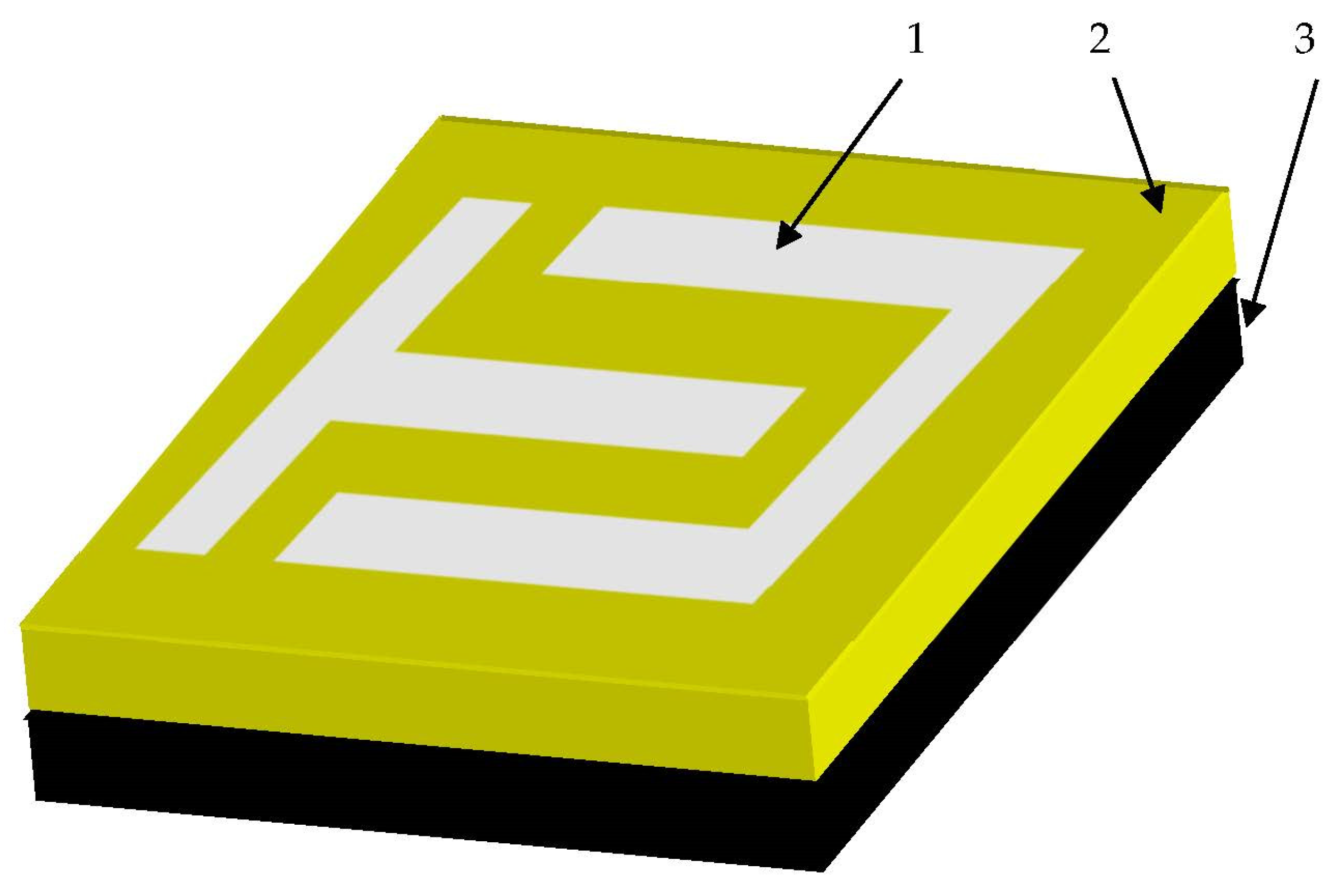
3.1. Carbon Substrates
- (1)
- A micro-structured carbon substrate with a surface pattern, with a thickness of about 10 micrometers;
- (2)
- Organic material: heterocyclic aromatic compounds, with a thickness between 0.1 and 2.0 micrometers;
- (3)
- Electro-conducting-painted comb type contact, tape thickness, width, and length were 10.0 micrometers, 1 mm, and 3 mm, respectively.
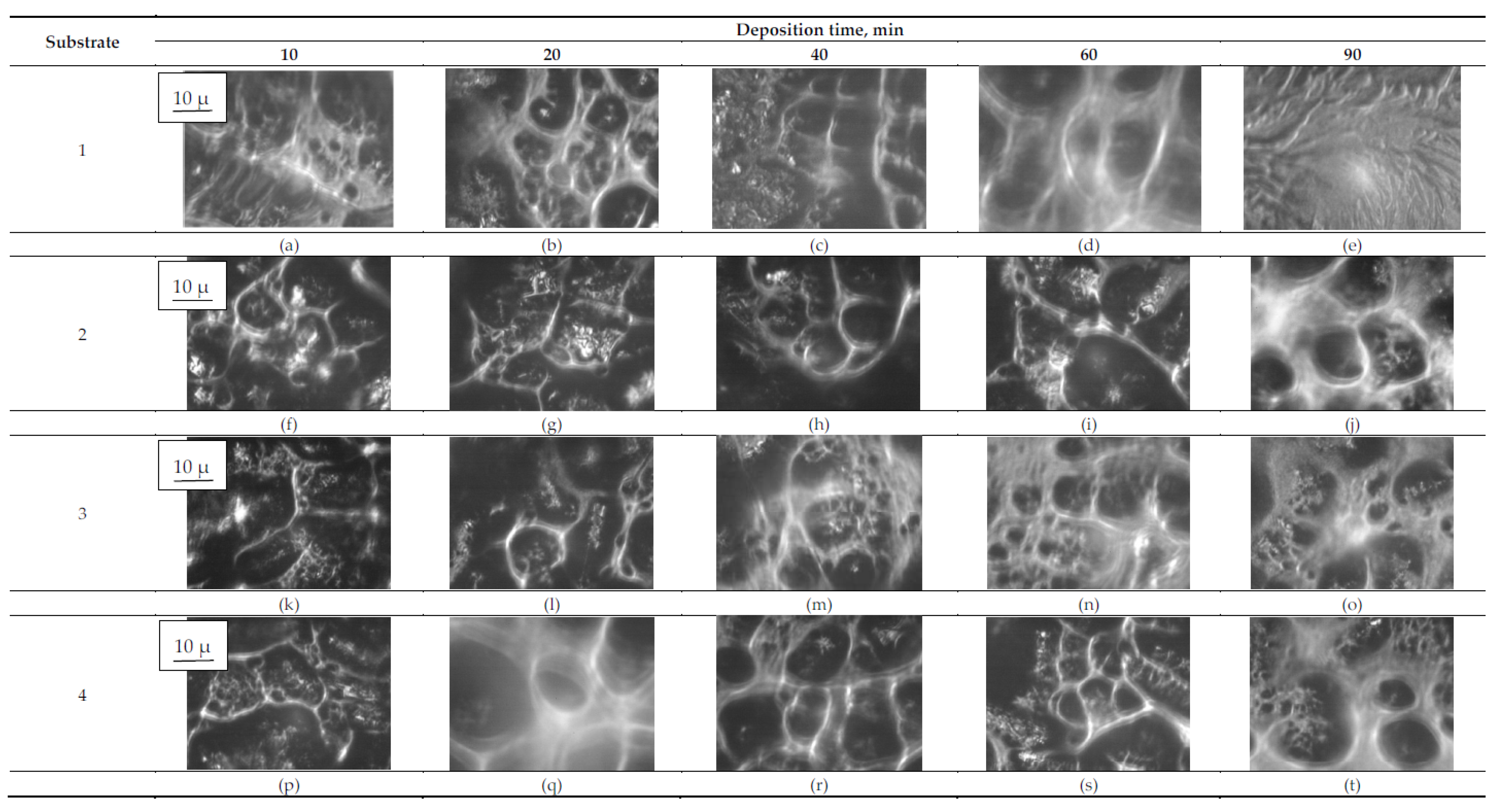
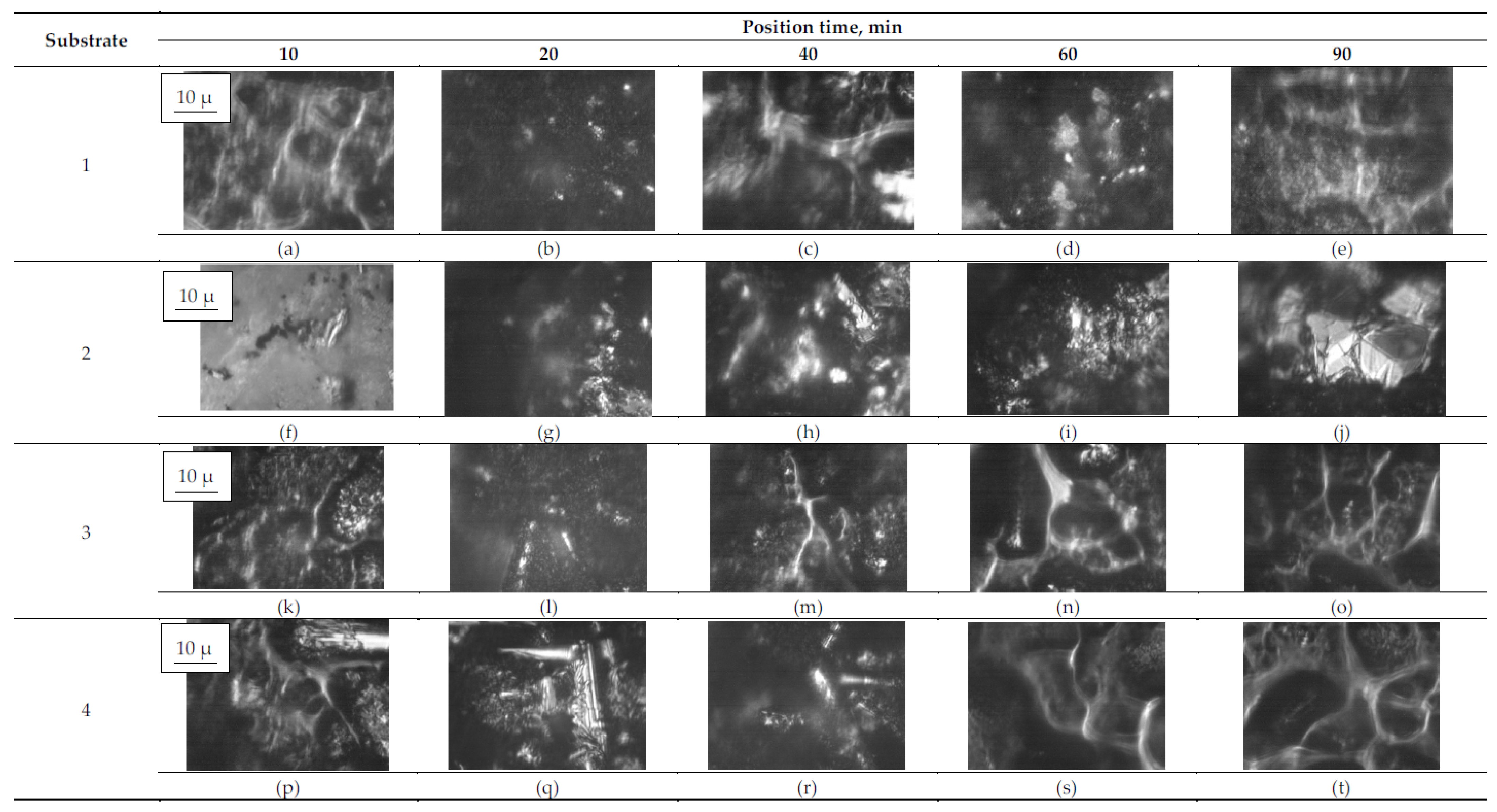
3.2. Surface Morphology
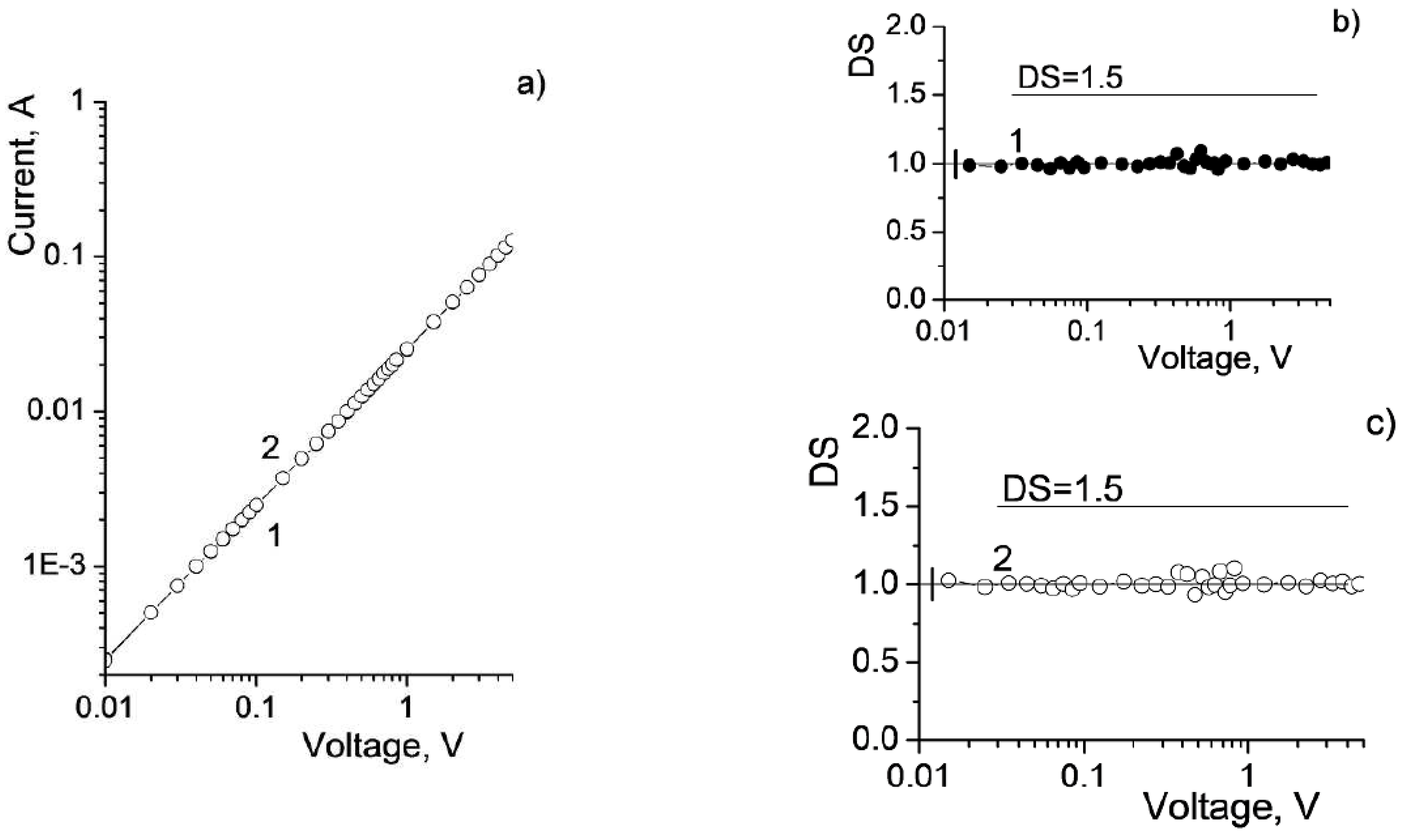
3.3. Current–Voltage Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goodwin, B. Evolution: Self-organization Theory. Int. Encycl. Soc. Behav. Sci. 2001, 5003–5007. [Google Scholar] [CrossRef]
- Gorbach, T.Y.; Smertenko, P.S.; Venger, E.F. Investigation of photovoltaic and optical properties of self-organized organic-inorganic hybrids using aromatic drugs and patterned silicon. Ukr. J. Phys. 2014, 59, 601–611. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Jiang, X.; Ni, Q.; Liu, C.; Shang, F.; Xia, Q.; Zhang, S. Self-Assembly at a Macroscale Using Aerodynamics. Appl. Sci. 2022, 12, 7676. [Google Scholar] [CrossRef]
- Aguirre, J.C.; Ferreira, A.; Ding, H.; Jenekhe, S.A.; Kopidakis, N.; Asta, M.; Pilon, L.; Rubin, Y.; Tolbert, S.H.; Schwartz, B.J.; et al. Panoramic View of Electrochemical Pseudocapacitor and Organic Solar Cell Research in Molecularly Engineered Energy Materials (MEEM). J. Phys. Chem. C 2014, 118, 19505–19523. [Google Scholar] [CrossRef] [Green Version]
- Tao, F. and Bernasek, S.L. Functionalization of Semiconductor Surfaces; Wiley: Hoboken, NJ, USA, 2012; p. 454. [Google Scholar]
- Ahoulou, S.; Perret, E.; Nedelec, J.-M. Functionalization and Characterization of Silicon Nanowires for Sensing Applications: A Review. Nanomaterials 2021, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Grynko, D.A.; Fedoryak, A.N.; Smertenko, P.S.; Dimitriev, O.P.; Ogurtsov, N.A.; Pud, A.A. Hybrid solar cell on a carbon fiber, Hybrid solar cell on a carbon fiber. Nanoscale Res. Lett. 2016, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Fagiolari, L.; Bella, F. Carbon-based materials for stable, cheaper and large-scale processable perovskite solar cells. Energy Environ. Sci. 2019, 12, 3437–3472. [Google Scholar] [CrossRef] [Green Version]
- Selvaganesh, S.; Bhat, S.D. Insight towards Nucleation Mechanism and Change in Morphology for Nanostructured Platinum Thin Film Directly Grown on Carbon Substrate via Electrochemical Deposition. Materials 2021, 14, 2330. [Google Scholar] [CrossRef]
- Rajamohan, N.; SaidAl Shibli, F.S.Z. Synthesis and application of carbon substrate nano material from biomass for surface protection—Effect of variables, electrochemical and isotherm studies. Chemosphere 2022, 292, 133479. [Google Scholar] [CrossRef]
- Ramesh, M.; Rajeshkumar, L.; Bhoopathi, R. Carbon substrates: A review on fabrication, properties and applications. Carbon Lett. 2021, 31, 557–580. [Google Scholar] [CrossRef]
- Bogachuk, D.; Yang, B.; Suo, J.; Martineau, D.; Verma, A.; Narbey, S.; Anaya, M.; Frohna, K.; Doherty, T.; Müller, D.; et al. Perovskite Solar Cells with Carbon-Based Electrodes—Quantification of Losses and Strategies to Overcome Them. Adv. Energy Mater. 2022, 12, 2103128. [Google Scholar] [CrossRef]
- Muchuweni, E.; Mombeshora, E.T.; Martincigh, B.S.; Nyamori, V.O. Recent Applications of Carbon Nanotubes in Organic Solar Cells. Front. Chem. 2021, 9, 733552. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, Y.-T.; Zhang, W.-S.; Zhang, X.-F.; Yin, X.; Li, J.; Wu, G.-P. Rational design of carbon network structure in microporous layer toward enhanced mass transport of proton exchange membrane fuel cell. J. Power Sources 2022, 539, 231623. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Wang, H.; Han, X.; Zhang, S.; Sun, J.; Zhang, Y.; Cao, Y.; Yao, Y.; Sun, J. Synthesis of greenish phosphorus on carbon substrates. Chem. Commun. 2021, 33, 3975–3978. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Z.-L.; Yang, J.-Q.; Liang, B.; Lin, X.-Q.; Nan, J.; Wang, A.-J. Effects of different carbon substrates on performance, microbiome community structure and function for bioelectrochemical-stimulated dechlorination of tetrachloroethylene. Chem. Eng. J. 2018, 352, 730–736. [Google Scholar] [CrossRef]
- Lockett, M.R.; Smith, L.M. Carbon Substrates: A Stable Foundation for Biomolecular Arrays. Annu. Rev. Anal. Chem. 2015, 8, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Belouet, C.; Monville, M.; Bigot, C.; Jolivet, E.; Varrot, R.; Chancolon, J.; Bonnamy, S. The carbon substrate in RST Si ribbon technology for solar cells. Carbon 2019, 141, 427–443. [Google Scholar] [CrossRef]
- Chu, H.; Zhang, Z.; Liu, Y.; Leng, J. Self-heating fiber reinforced polymer composite using meso/macropore carbon nanotube paper and its application in deicing. Carbon 2014, 66, 154–163. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.D.P.S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T., Jr.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and grapheme. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y.; Aksas, H. Carbon Nanotubes (CNTs) from Synthesis to Functionalized (CNTs) Using Conventional and New Chemical Approaches. J. Nanomater. 2021, 2021, 4972770. [Google Scholar] [CrossRef]
- Chaudhary, K.T. Thin Film Deposition: Solution Based Approach. In Thin Films; Ares, A.E., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Wang, H.; Frontera, C.; Herrero-Martín, J.; Pomar, A.; Roura, P.; Martínez, B.; Mestres, N. Aqueous Chemical Solution Deposition of Functional Double Perovskite Epitaxial Thin Films. Chem. A Eur. J. 2020, 26, 9338–9347. [Google Scholar] [CrossRef] [PubMed]
- Smertenko, P.S.; Roshchina, N.M.; Kuznetsova, D.A.; Barsukov, V.Z.; Wisz, G. Vitamin B12-functionalized patterned Si surface for solar energy conversion. SPQEO 2018, 21, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Inigo, A.R.; Xavier, F.P.; Goldsmith, G.J. Copper phthalocyanine as an efficient dopant in development of solar cells. Mater. Res. Bull. 1997, 32, 539–546. [Google Scholar] [CrossRef]
- Salzman, R.F.; Xue, J.; Rand, B.; Alexander, A.; Thompson, M.E.; Forrest, S.R. The effects of copper phthalocyanine purity on organic solar cell performance. Org. Electron. 2005, 6, 242–246. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, R.S.; Parthasarathy, B.; Aguilera, A.; Anthony, J.; Payne, M. Copper-phthalocyanine-based organic solar cells with high open-circuit voltage. Appl. Phys. Lett. 2005, 86, 082106. [Google Scholar] [CrossRef]
- Luong, T.T.T.; Chen, Z.; Zhu, H. Flexible solar cells based on copper phthalocyanine and buckminsterfullerene. Sol. Energy Mater. Sol. Cells 2010, 94, 1059–1063. [Google Scholar] [CrossRef]
- Pindolia, G.; Pandya, J.; Shinde, S.; Jha, P.K. Fluorinated copper phthalocyanine as an electron transport material in perovskite solar cell. Int. J. Energy Res. 2022, 46, 15127–15142. [Google Scholar] [CrossRef]
- Wittbrodt, E.T.; Pharm, D. Drugs and Myasthenia Gravis. Arch. Intern. Med. 1997, 157, 399–408. [Google Scholar] [CrossRef]
- Ciach, R.; Dotsenko, Y.; Naumov, V.; Shmyryeva, A.; Smertenko, P. Injection Technique for Study of Solar Cells Test Structures. Sol. Energy Mater. Sol. Cells 2003, 76, 613–624. [Google Scholar] [CrossRef]
- Barsukov, I.V.; Gallego, M.A.; Doninger, J.E. Novel materials for electrochemical power sources—Introduction of PUREBLACK® Carbons. J. Power Sources 2006, 153, 288–299. [Google Scholar] [CrossRef]
- Barsukov, V.; Senyk, I.; Kryukova, O.; Butenko, O. Composite Carbon-Polymer Materials for Electromagnetic Radiation Shielding. Mater. Today Proc. 2018, 5, 15909–15914. [Google Scholar] [CrossRef]
- Butenko, O.; Boychuk, V.; Savchenko, B.; Kotsyubynsky, V.; Khomenko, V.; Barsukov, V. Pure ultrafine magnetite from carbon steel wastes. Mater. Today Proc. 2019, 6, 270–278. [Google Scholar] [CrossRef]
- Budko, O.; Butenko, O.; Chernysh, O.; Khomenko, V.; Tverdokhlib, V.; Barsukov, V. Effect of grain composition of natural graphites on electrical conductivity of graphite-based composite materials. J. Mater. Today Proc. 2021, 50, 535–538. [Google Scholar] [CrossRef]



| N | Title | Molecular Formula | Additional Substances |
|---|---|---|---|
| 1 | Sulfacyl sodium, sulfanilamide, C8H10N2O3S | 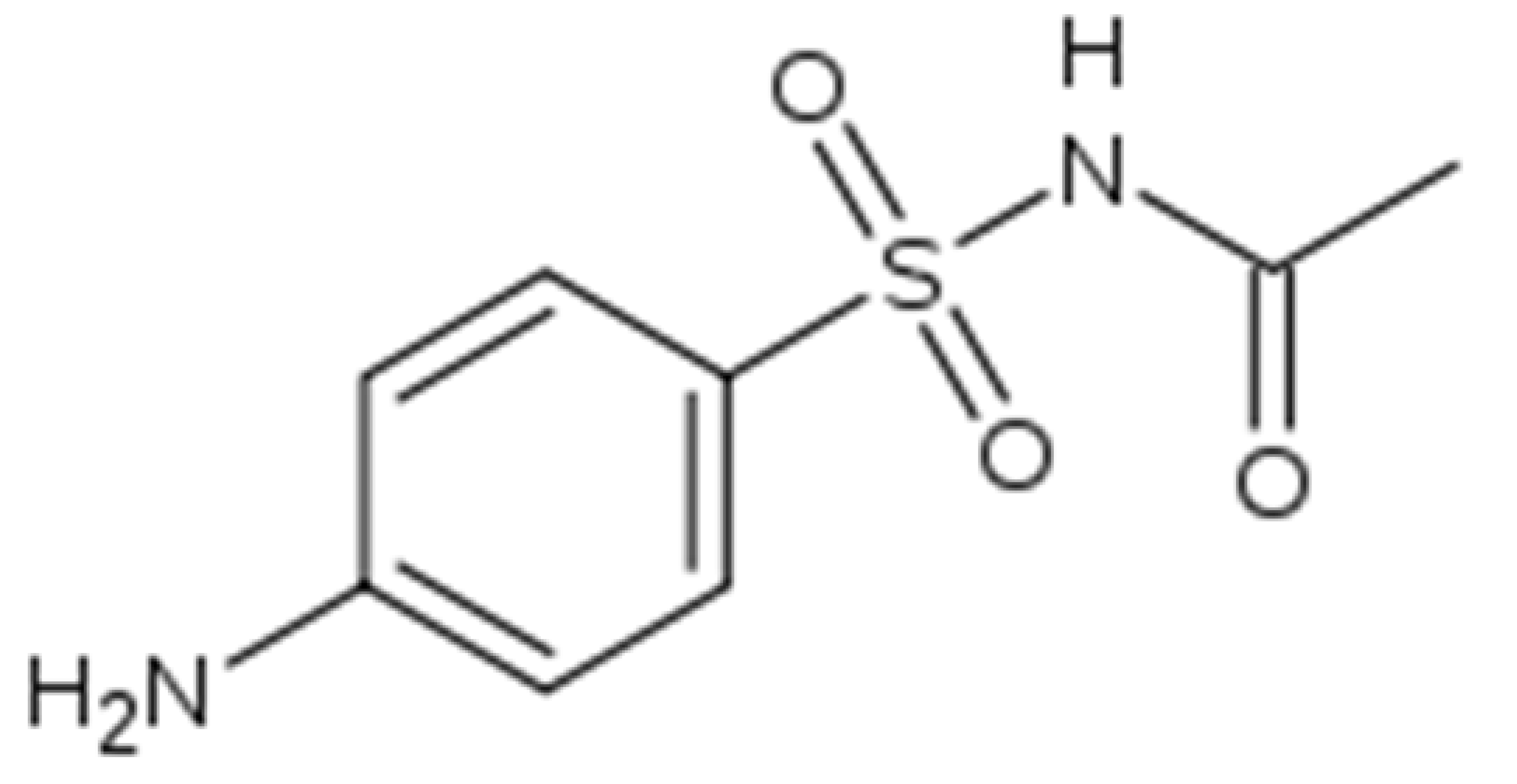 | H2O |
| 2 | Clonidine hydrochloride, C9H9Cl2N3, (N-(2,6-dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine) | 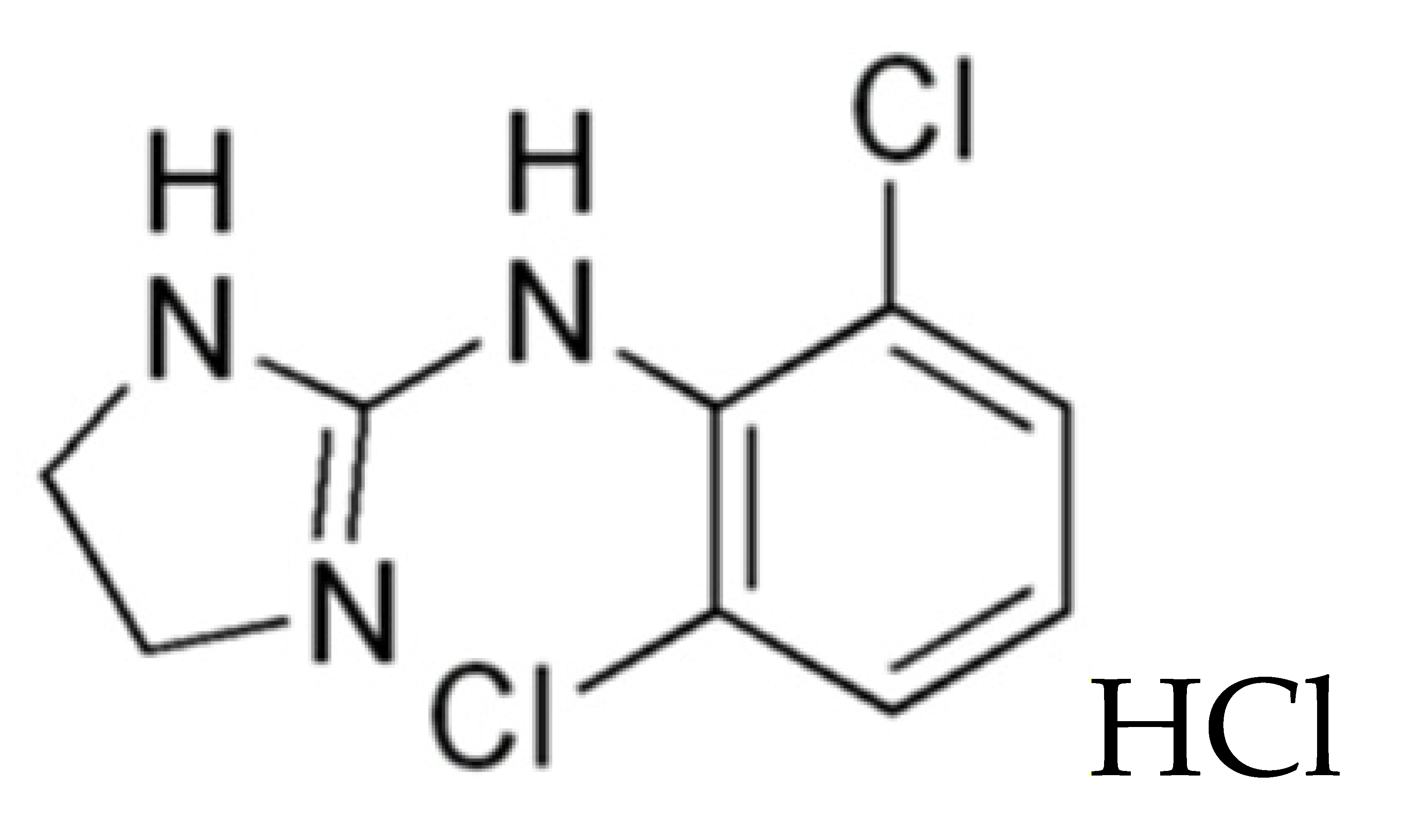 HCl HCl | Tablet: lactose monohydrate, starch, polyvidone, magnesium stearate. H2O |
| 3 | Cyanocobalamin (Vitamin B12), C63H88CoN14O14P, Coα-[α-(5.6 dimethyl benzimidazolile-Coβ-cobamidcyanide |  | H2O |
| No. of Substrate | Resistivity, Ohm | Composition | Surface Morphology | |
|---|---|---|---|---|
| Electronic Image | Optical Image | |||
| 1 | 4.0 | 20% carbon black 20% activated carbon (compressed) 40% graphite KGPS-1 20% PVB |  | 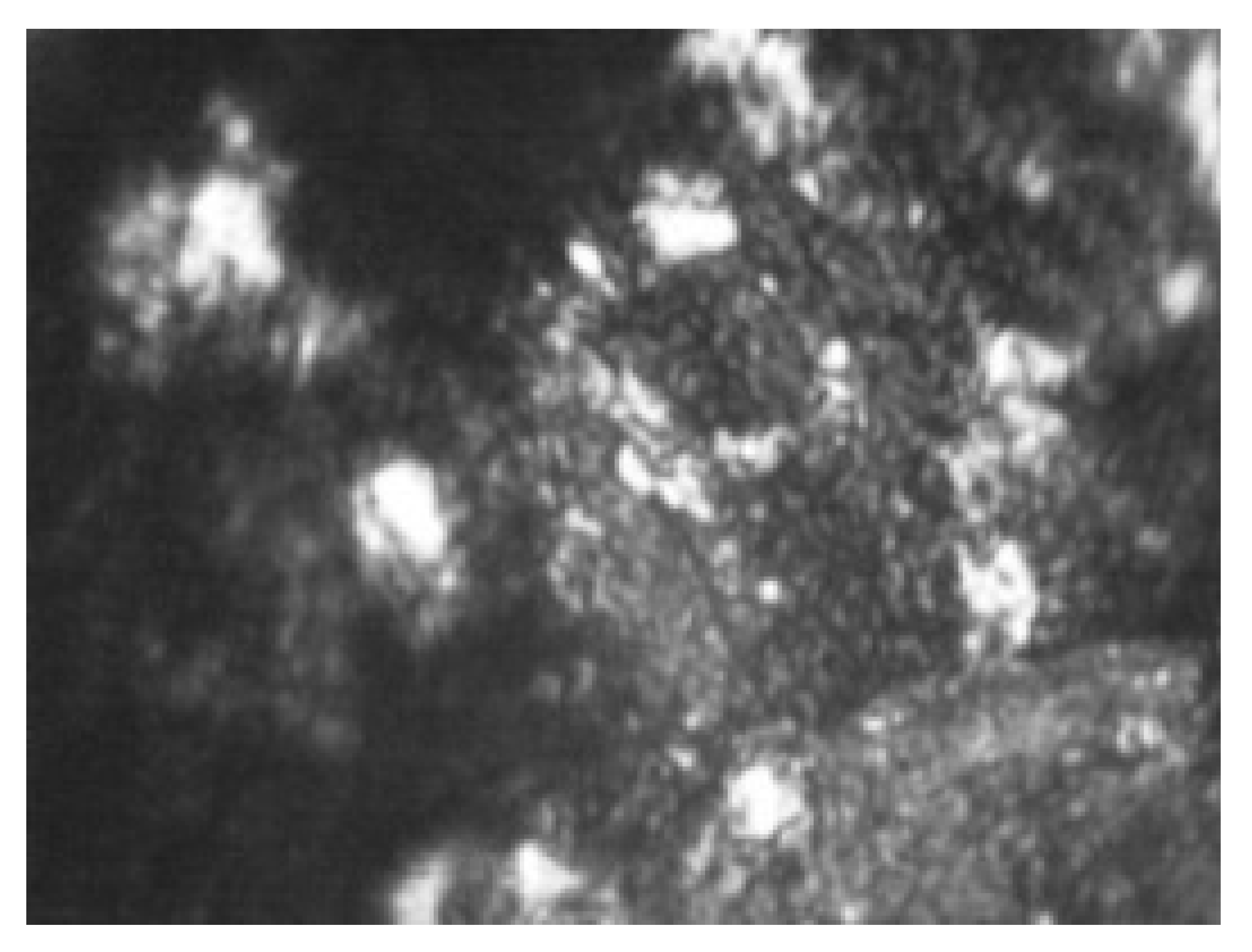 |
| 2 | 3.6 | 16,67% carbon black 50% graphite KGPS-1 16,67% magnetite (BM) 20% PVB | 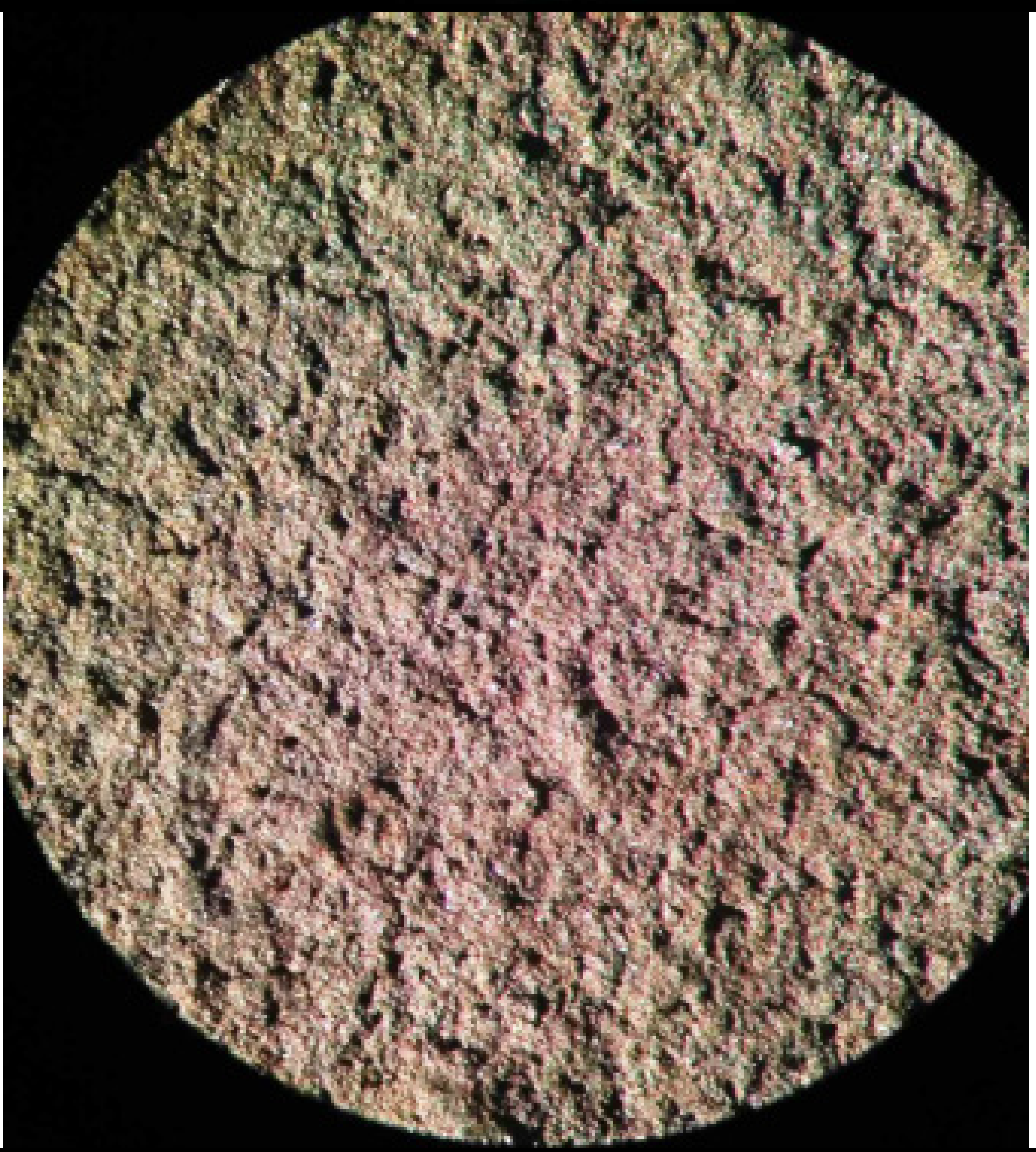 |  |
| 3 | 26.4 | 20% carbon black 50% graphite KGPS-1 10% carbon nanotubes 20 % PVB | 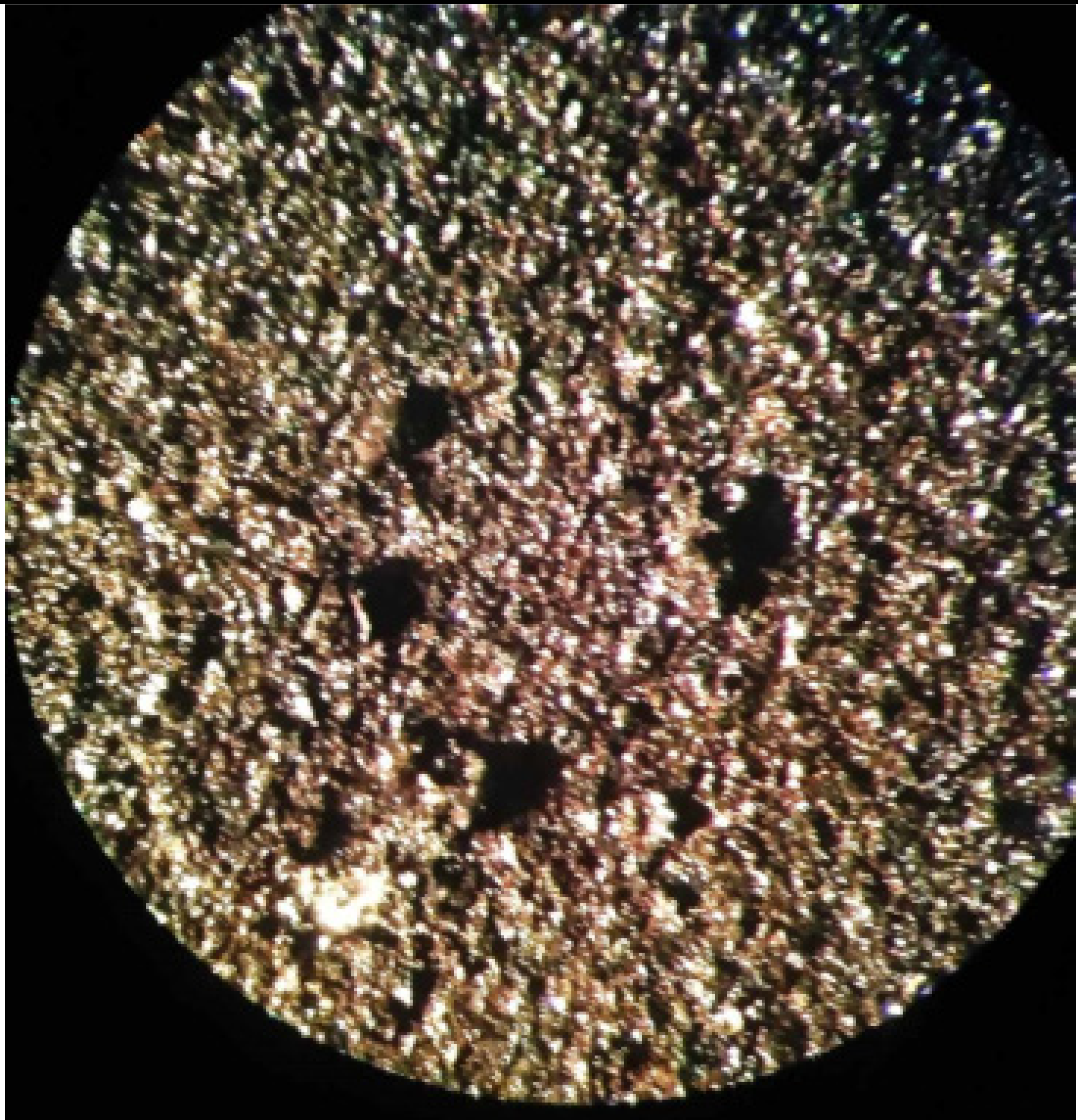 |  |
| 4 | 3.0 | 20% carbon black 40% graphite KGPS-1 20% graphite DBX-010 20% PVB |  | 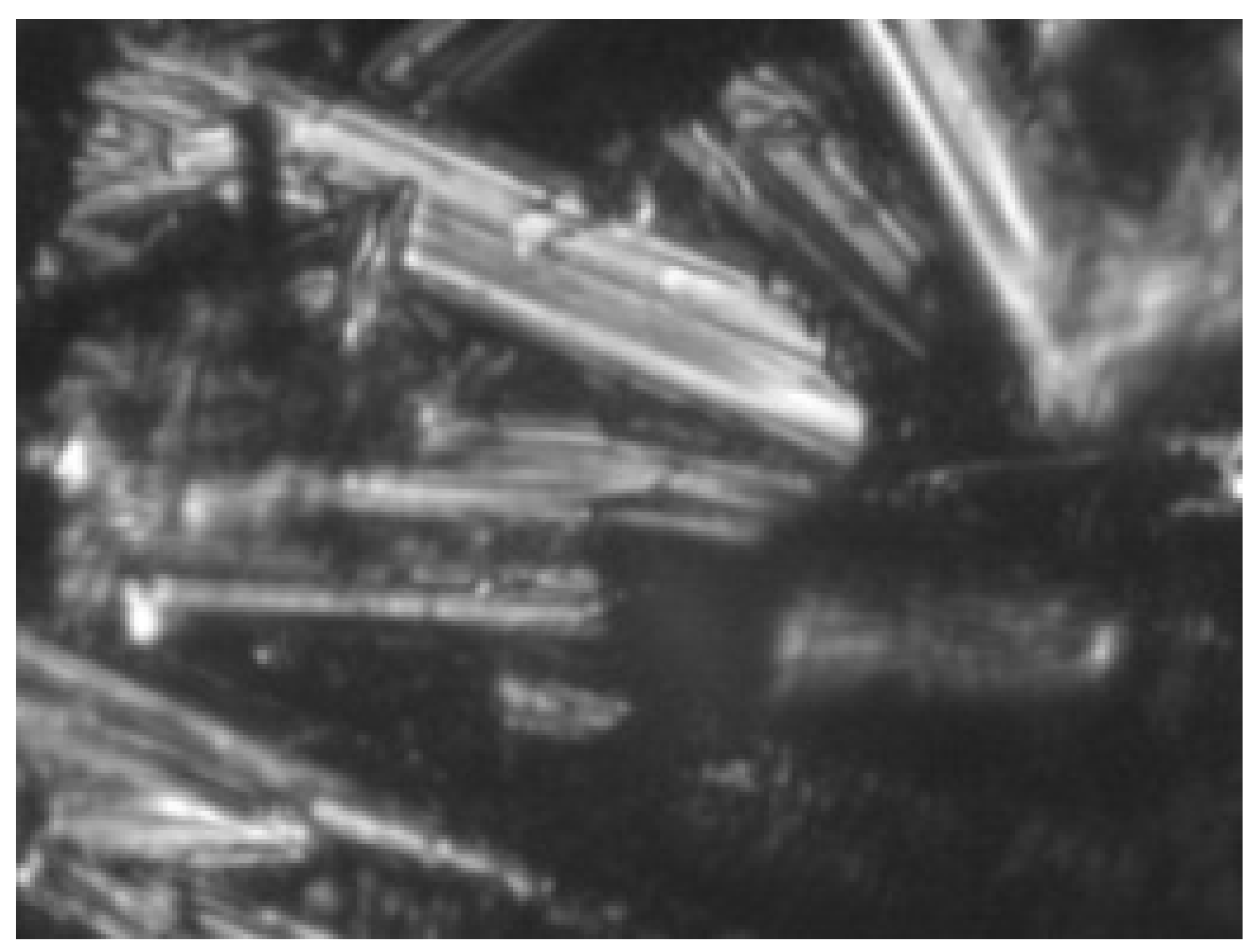 |
| Substrate | Deposition Time | ||||
|---|---|---|---|---|---|
| 10 min | 20 min | 40 min | 60 min | 90 min | |
| Sulfacyl sodium | |||||
| 1 | 2.04 | 2.28 | 1.45 | 1.16 | 1.43 |
| 2 | 1.0 | 1.12 | 1.03 | 1.0 | 1.73 |
| 3 | 1.58 | 1.25 | 1.71 | 2.03 | 1.28 |
| 4 | 1.99 | 1.0 | 1.67 | 1.02 | 2.82 |
| Clonidine | |||||
| 1 | 1.38 | 3.68 | 2.25 | 1.46 | 1.0 |
| 2 | 1.04 | 1.06 | 1.0 | 1.0 | 1.0 |
| 3 | 1.15 | 1.0 | 1.07 | 1.16 | 1.09 |
| 4 | 1.16 | 1.13 | 1.26 | 1.1 | 1.45 |
| Vitamin B12 | |||||
| 1 | 1.99 | 1.0 | 1.14 | 1.0 | 1.05 |
| 2 | 1.0 | 1.01 | 1.1 | 1.04 | 1.13 |
| 3 | 1.04 | 1.02 | 1.06 | 1.0 | 1.01 |
| 4 | 1.0 | 1.0 | 1.0 | 1.5 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smertenko, P.; Roshchina, N.; Olkhovik, G.; Khomenko, V.; Butenko, O.; Chernysh, O.; Barsukov, V. Self-Organized Heterocyclic Amines Films on Carbon Substrates for Photovoltaic Applications. Appl. Sci. 2022, 12, 10210. https://doi.org/10.3390/app122010210
Smertenko P, Roshchina N, Olkhovik G, Khomenko V, Butenko O, Chernysh O, Barsukov V. Self-Organized Heterocyclic Amines Films on Carbon Substrates for Photovoltaic Applications. Applied Sciences. 2022; 12(20):10210. https://doi.org/10.3390/app122010210
Chicago/Turabian StyleSmertenko, Petro, Nina Roshchina, Gennadiy Olkhovik, Volodymyr Khomenko, Oksana Butenko, Oksana Chernysh, and Viacheslav Barsukov. 2022. "Self-Organized Heterocyclic Amines Films on Carbon Substrates for Photovoltaic Applications" Applied Sciences 12, no. 20: 10210. https://doi.org/10.3390/app122010210






