Estimating the Margin of Gait Stability in Healthy Elderly Using the Triaxial Kinematic Motion of a Single Body Feature
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Used for Analysis
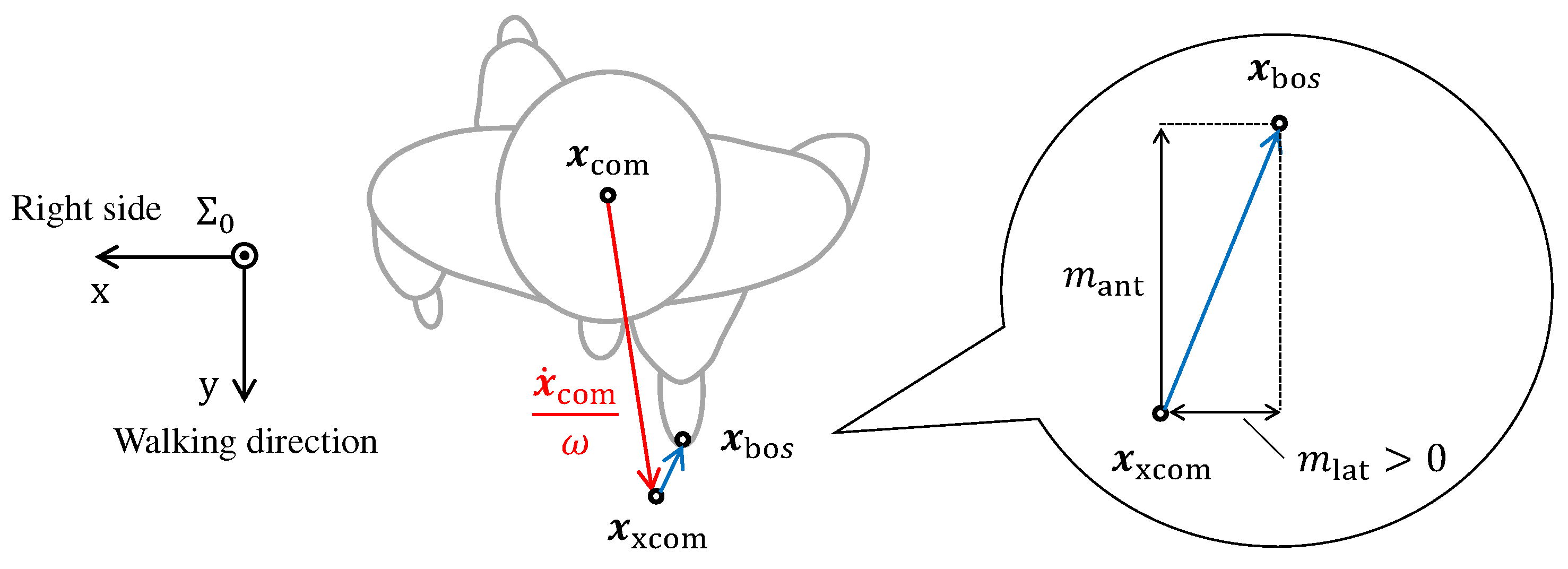
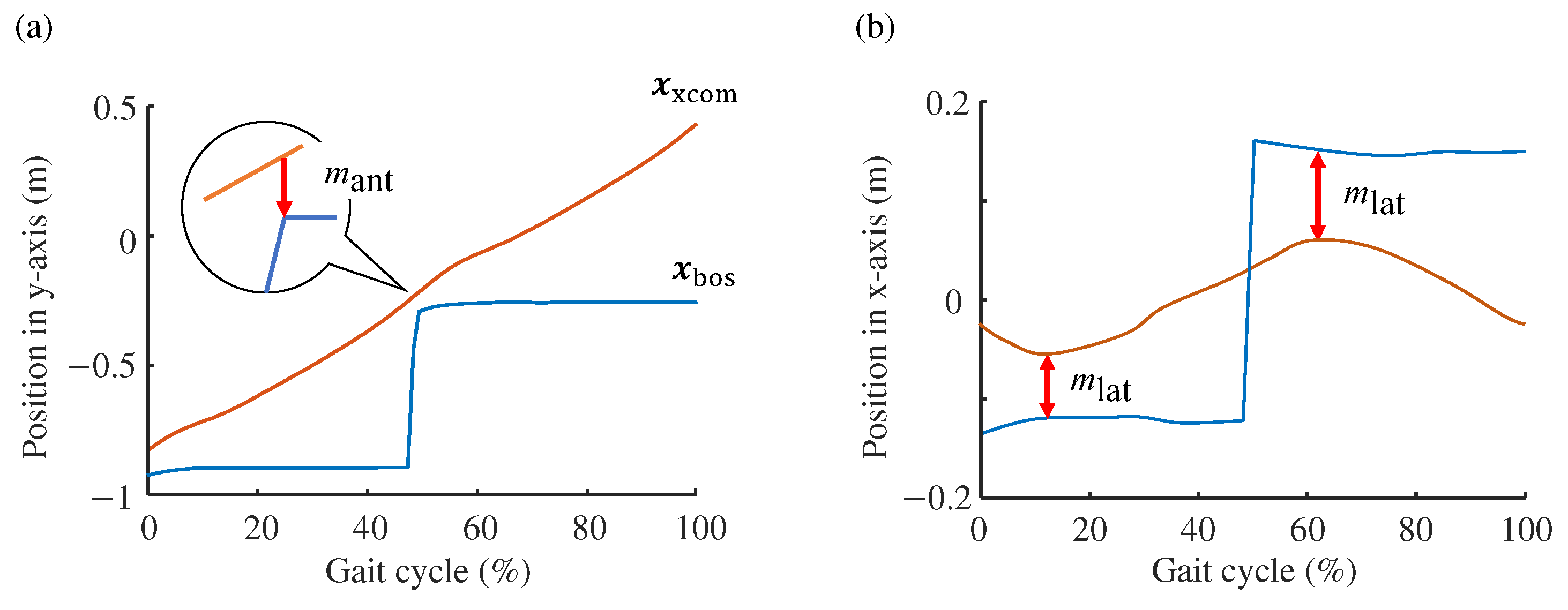
2.2. Computation of Margin of Stability
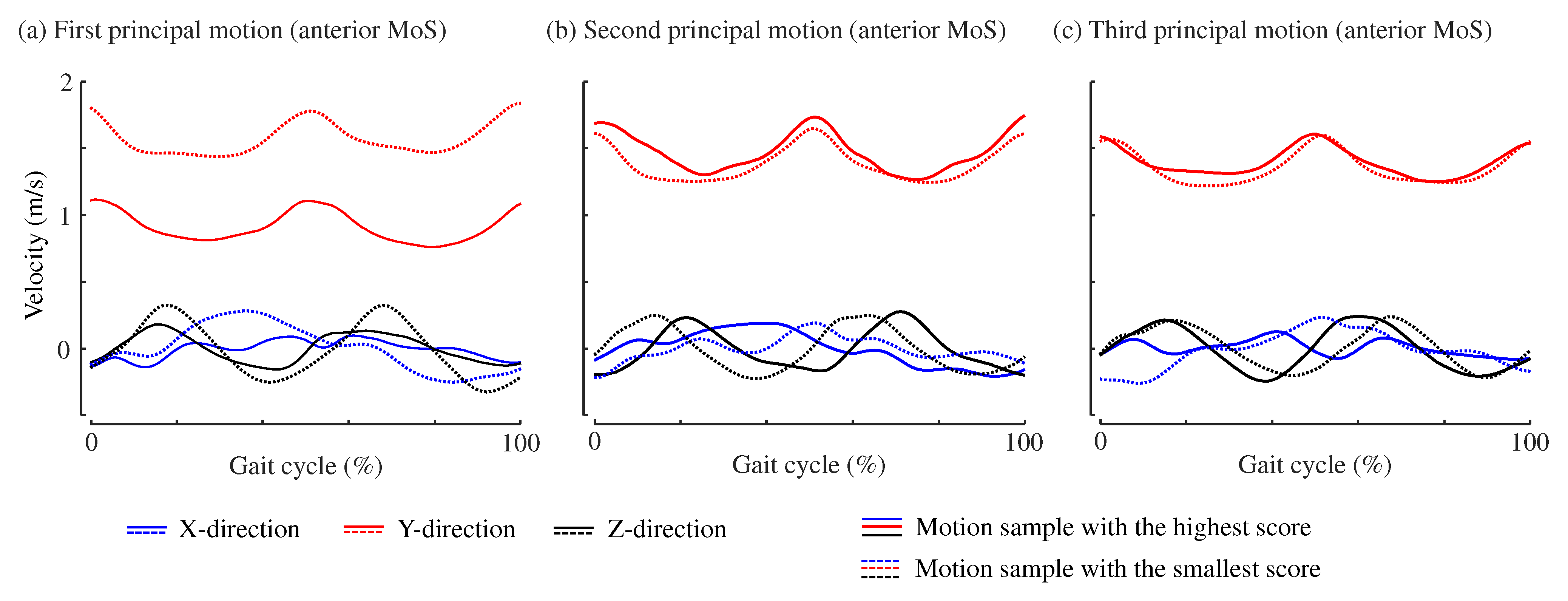
2.3. Principal Motion Analysis to Estimate MoS Values from Kinematic Data
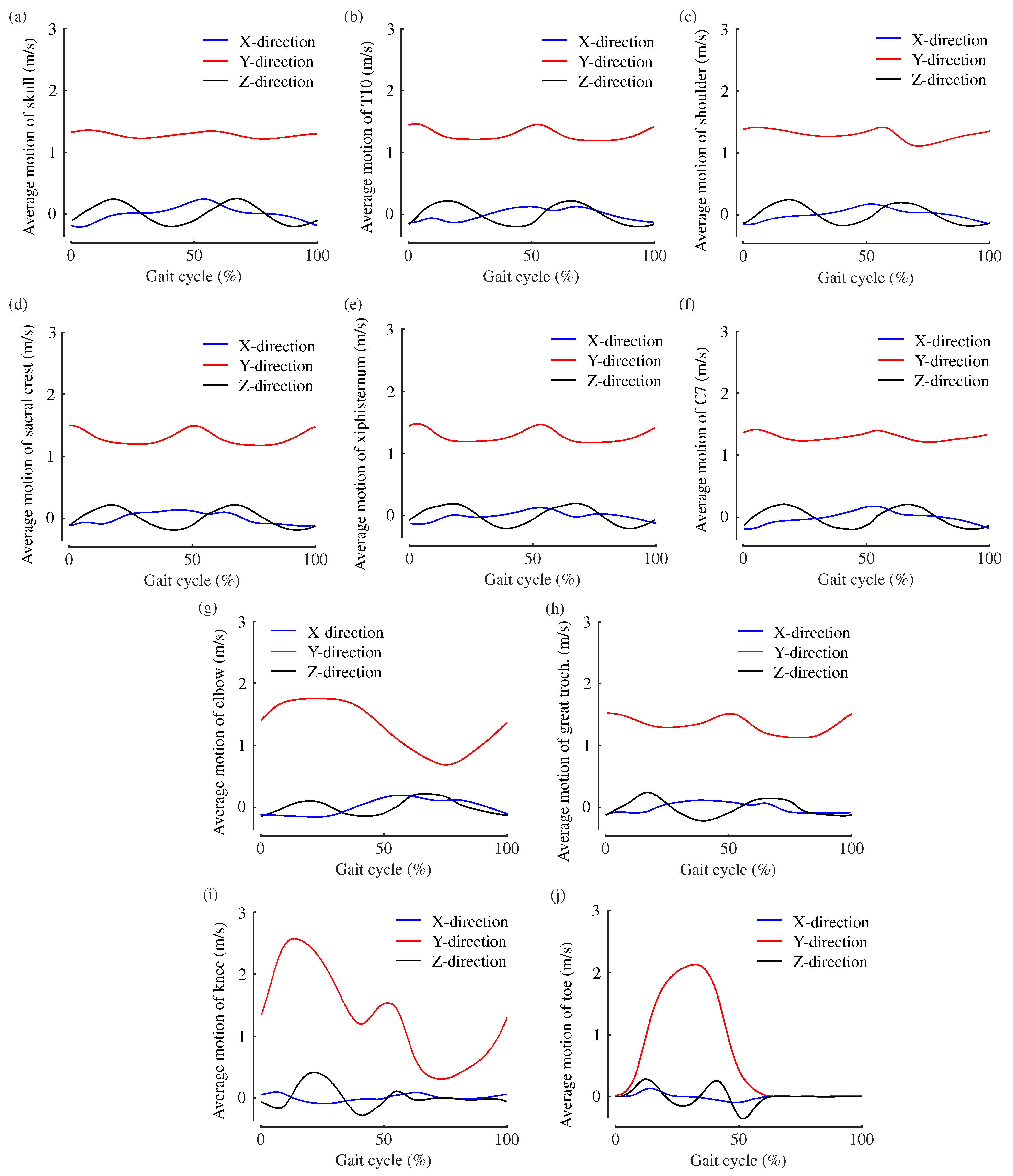
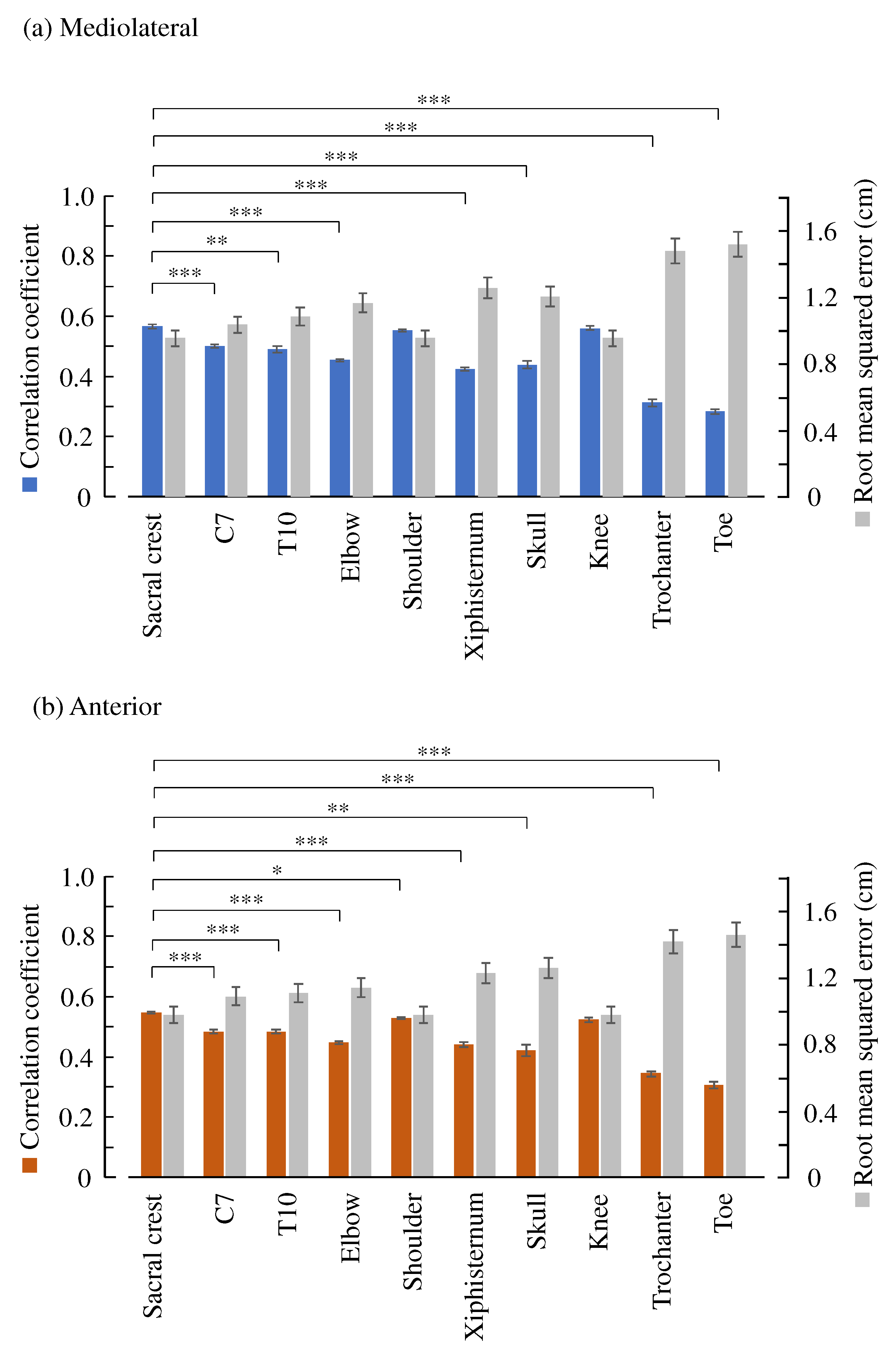
2.4. Comparison of Ten Body Features
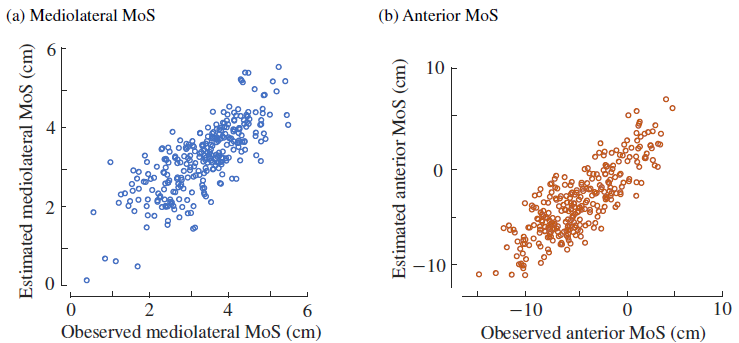
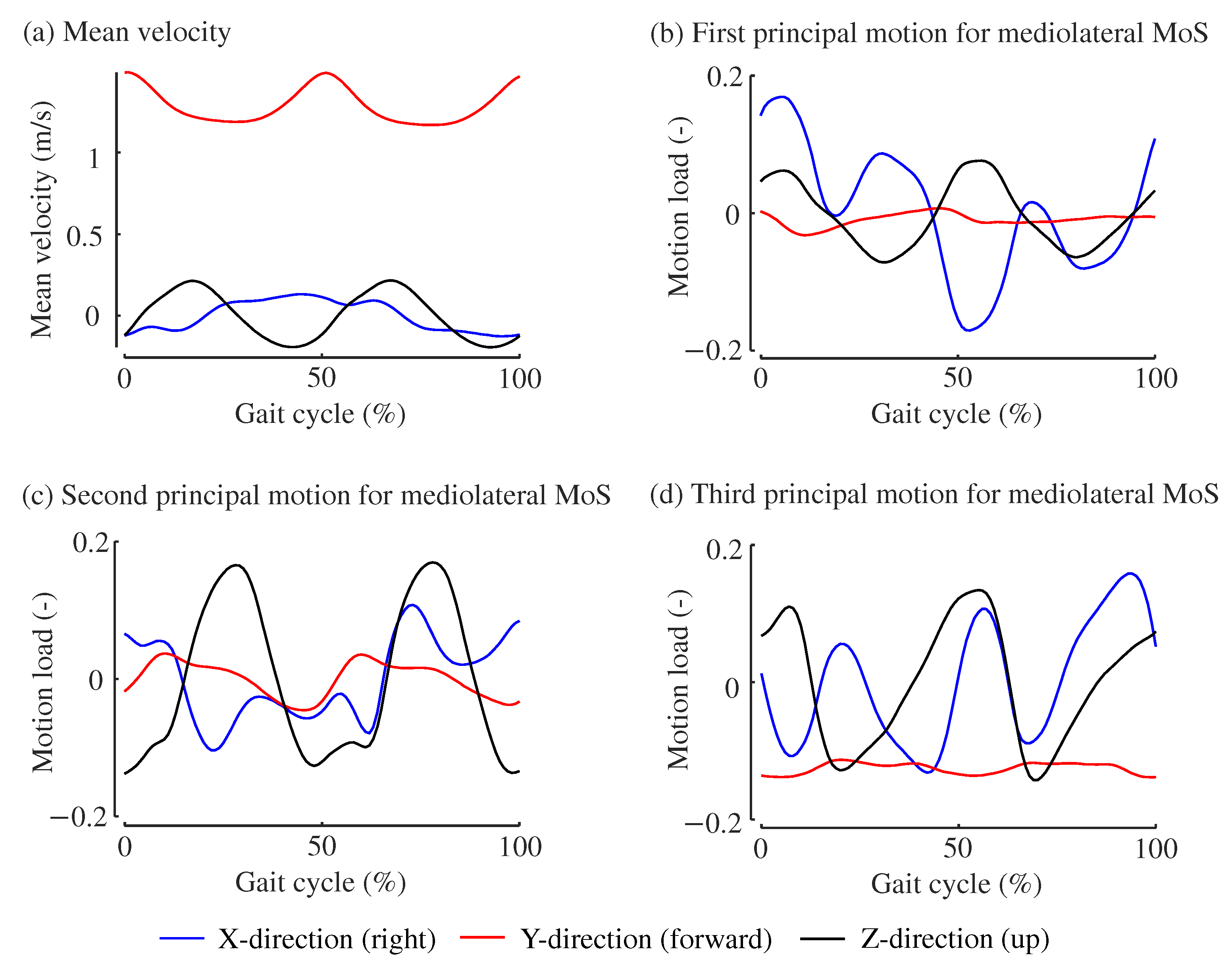
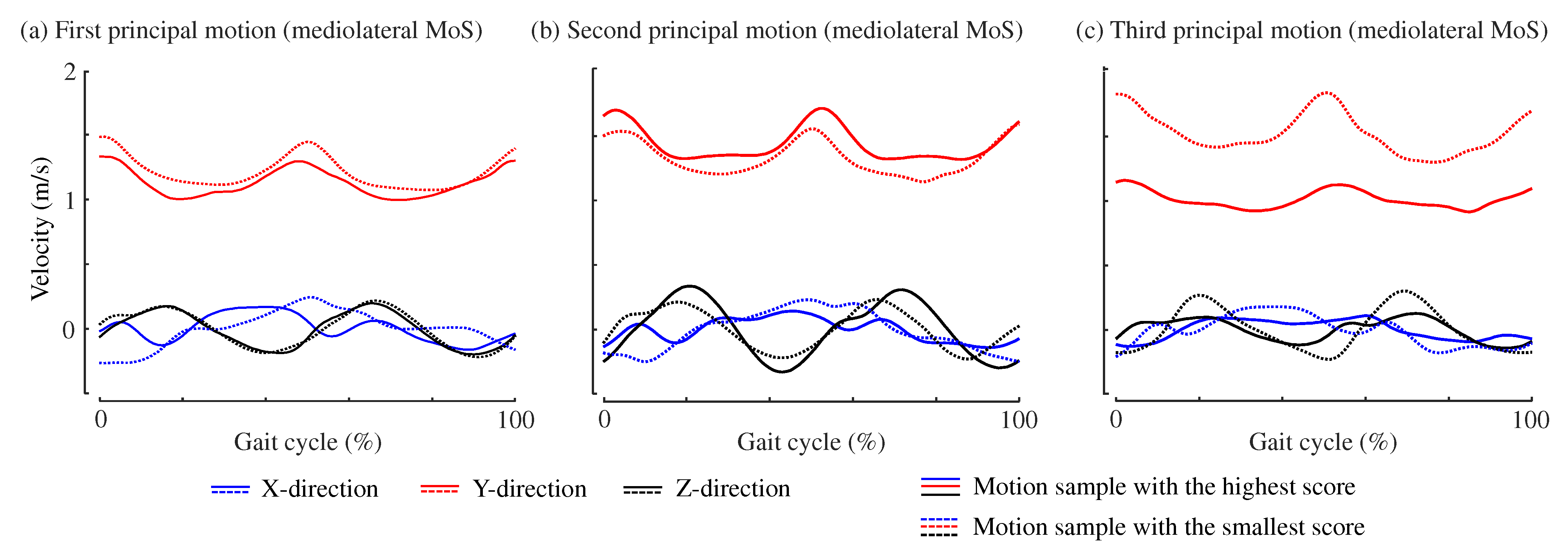
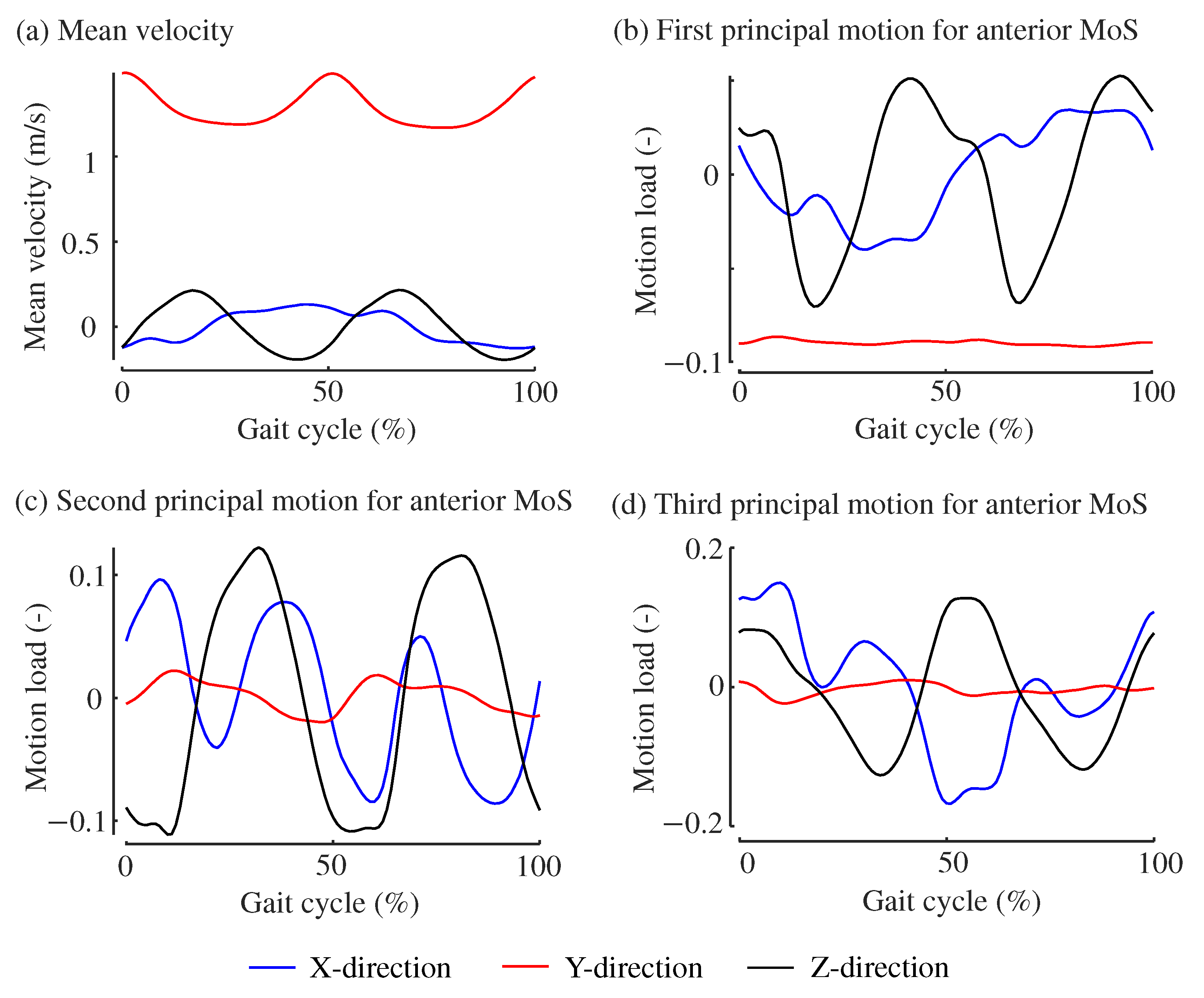
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MoS | Margin of stability |
| IMU | Inertial measurement unit |
| PMA | Principal motion analysis |
References
- Balance Augmentation in Locomotion, through Anticipative, Natural and Cooperative Control of Exoskeletons; Report of BALANCE-Deliverable 3.1-Stability index 2013; European Commision: Brussels, Belgium, 2013.
- Bruijn, S.; Meijer, O.; Beek, P.; van Dieën, J. Assessing the stability of human locomotion: A review of current measures. J. R. Soc. Interface 2013, 10, 20120999. [Google Scholar] [CrossRef]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Sherrington, C.; Gates, S.; Clemson, L.; Lamb, S.E. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2012, 2021, CD007146. [Google Scholar] [CrossRef]
- Kim, Y.K.; Joo, J.Y.; Jeong, S.H.; Jeon, J.H.; Jung, D.Y. Effects of walking speed and age on the directional stride regularity and gait variability in treadmill walking. J. Mech. Sci. Technol. 2016, 30, 2899–2906. [Google Scholar] [CrossRef]
- Liu, T.; Inoue, Y.; Shibata, K. Development of a wearable sensor system for quantitative gait analysis. Measurement 2009, 42, 978–988. [Google Scholar] [CrossRef]
- Jebelli, H.; Ahn, C.R.; Stentz, T.L. Comprehensive Fall-Risk Assessment of Construction Workers Using Inertial Measurement Units: Validation of the Gait-Stability Metric to Assess the Fall Risk of Iron Workers. J. Comput. Civ. Eng. 2016, 30, 04015034. [Google Scholar] [CrossRef]
- Mariani, B.; Hoskovec, C.; Rochat, S.; Büla, C.; Penders, J.; Aminian, K. 3D gait assessment in young and elderly subjects using foot-worn inertial sensors. J. Biomech. 2010, 43, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Jebelli, H.; Ahn, C.R.; Stentz, T.L. Fall risk analysis of construction workers using inertial measurement units: Validating the usefulness of the postural stability metrics in construction. Saf. Sci. 2016, 84, 161–170. [Google Scholar] [CrossRef]
- Yang, K.; Ahn, C.R.; Vuran, M.C.; Aria, S.S. Semi-supervised near-miss fall detection for ironworkers with a wearable inertial measurement unit. Autom. Constr. 2016, 68, 194–202. [Google Scholar] [CrossRef]
- Riek, P.M.; Best, A.N.; Wu, A.R. Validation of Inertial Sensors to Evaluate Gait Stability. Sensors 2023, 23, 1547. [Google Scholar] [CrossRef]
- Hof, A.; Gazendam, M.; Sinke, W. The condition for dynamic stability. J. Biomech. 2005, 38, 1–8. [Google Scholar] [CrossRef]
- Hof, A.L. The ‘extrapolated center of mass’ concept suggests a simple control of balance in walking. Hum. Mov. Sci. 2008, 27, 112–125. [Google Scholar] [CrossRef]
- Watson, F.; Fino, P.C.; Thornton, M.; Heracleous, C.; Loureiro, R.; Leong, J.J.H. Use of the margin of stability to quantify stability in pathologic gait—A qualitative systematic review. BMC Musculoskelet. Disord. 2021, 22, 597. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kuboki, Y.; Okamoto, S.; Yamada, Y. Novel Approach to Analyze All-Round Kinematic Stability during Curving Steps. IEEE Access 2023, 11, 10326–10335. [Google Scholar] [CrossRef]
- Ohtsu, H.; Yoshida, S.; Minamisawa, T.; Takahashi, T.; Yomogida, S.i.; Kanzaki, H. Investigation of balance strategy over gait cycle based on margin of stability. J. Biomech. 2019, 95, 109319. [Google Scholar] [CrossRef] [PubMed]
- Harro, C.; Alderink, G.; Hickox, L.; Zeitler, D.W.; Avery, M.; Daman, C.; Laker, D. Dynamic Measures of Balance during Obstacle-Crossing in Self-Selected Gait in Individuals with Mild-to-Moderate Parkinson’s Disease. Appl. Sci. 2024, 14, 1271. [Google Scholar] [CrossRef]
- Kuroda, T.; Okamoto, S.; Akiyama, Y. Anterior and mediolateral dynamic gait stabilities attributed to different gait parameters in different age groups. J. Biomech. Sci. Eng. 2024, 19, 23–00183. [Google Scholar] [CrossRef]
- Nagano, H. Gait Biomechanics for Fall Prevention among Older Adults. Appl. Sci. 2022, 12, 6660. [Google Scholar] [CrossRef]
- Fallahtafti, F.; Bruijn, S.; Mohammadzadeh Gonabadi, A.; Sangtarashan, M.; Boron, J.B.; Curtze, C.; Siu, K.C.; Myers, S.A.; Yentes, J. Trunk Velocity Changes in Response to Physical Perturbations Are Potential Indicators of Gait Stability. Sensors 2023, 23, 2833. [Google Scholar] [CrossRef]
- Akiyama, Y.; Fukui, Y.; Okamoto, S.; Yamada, Y. Effects of exoskeletal gait assistance on the recovery motion following tripping. PLoS ONE 2020, 15, e0229150. [Google Scholar] [CrossRef] [PubMed]
- Alderink, G.; Harro, C.; Hickox, L.; Zeitler, D.W.; Bourke, M.; Gosla, A.; Rustmann, S. Dynamic Measures of Balance during a 90° Turn in Self-Selected Gait in Individuals with Mild Parkinson’s Disease. Appl. Sci. 2023, 13, 5428. [Google Scholar] [CrossRef]
- Varas-Diaz, G.; Jayakumar, U.; Taras, B.; Wang, S.; Bhatt, T. Assessing Balance Loss and Stability Control in Older Adults Exposed to Gait Perturbations under Different Environmental Conditions: A Feasibility Study. Biomechanics 2022, 2, 374–394. [Google Scholar] [CrossRef]
- Hak, L.; Hettinga, F.J.; Duffy, K.R.; Jackson, J.; Sandercock, G.R.; Taylor, M.J. The concept of margins of stability can be used to better understand a change in obstacle crossing strategy with an increase in age. J. Biomech. 2019, 84, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ko, Y.M.; Park, J.W. The Correlation between Dynamic Balance Measures and Stance Sub-phase COP Displacement Time in Older Adults during Obstacle Crossing. J. Phys. Ther. Sci. 2013, 25, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, S.; Schinkel-Ivy, A.; Masani, K.; Mansfield, A. Relationship between margin of stability and deviations in spatiotemporal gait features in healthy young adults. Hum. Mov. Sci. 2018, 57, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Okamoto, S.; Akiyama, Y.; Yamada, Y. Gait Stability Index Built by Kinematic Information Consistent with the Margin of Stability along the Mediolateral Direction. IEEE Access 2022, 10, 52832–52839. [Google Scholar] [CrossRef]
- Liu, Z.; Kuroda, T.; Okamoto, S.; Akiyama, Y. Estimation of Mediolateral Gait Postural Stability using Time-Series Pelvis Angular Velocities. In Proceedings of the IEEE Global Conference on Consumer Electronics, Osaka, Japan, 18–21 October 2022; pp. 754–756. [Google Scholar] [CrossRef]
- Qiu, C.; Okamoto, S.; Akiyama, Y.; Yamada, Y. Application of Supervised Principal Motion Analysis to Evaluate Subjectively Easy Sit-to-Stand Motion of Healthy People. IEEE Access 2021, 9, 73251–73261. [Google Scholar] [CrossRef]
- Liu, Z.; Kuroda, T.; Okamoto, S.; Akiyama, Y. Suitability of Sacrum Motion in Computing Dynamic Gait Stability Indices. In Proceedings of the IEEE Global Conference on Consumer Electronics, Nara, Japan, 10–13 October 2023; pp. 343–345. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hida, N.; Nakajima, K.; Fujimoto, M.; Mochimaru, M. AIST Gait Database 2019. Available online: https://unit.aist.go.jp/harc/ExPART/GDB2019.html (accessed on 1 June 2019).
- Yamaguchi, T.; Masani, K. Effects of age on dynamic balance measures and their correlation during walking across the adult lifespan. Sci. Rep. 2022, 12, 14301. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, T.; Kobayashi, Y.; Matsumoto, Y.; Murai, A.; Fujimoto, M.; Ozawa, J.; Mochimaru, M. Comparison of Machine Learning Methods and Gait Characteristics for Classification of Fallers and Non-fallers. Adv. Biomed. Eng. 2023, 12, 182–192. [Google Scholar] [CrossRef]
- Slijepcevic, D.; Horst, F.; Simak, M.; Lapuschkin, S.; Raberger, A.; Samek, W.; Breiteneder, C.; Schöllhorn, W.; Zeppelzauer, M.; Horsak, B. Explaining machine learning models for age classification in human gait analysis. Gait Posture 2022, 97, S252–S253. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Park, F.C.; Jo, K. Movement Primitives and Principal Component Analysis. In Proceedings of the on Advances in Robot Kinematics; Lenarčič, J., Galletti, C., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 421–430. [Google Scholar]
- Lim, B.; Ra, S.; Park, F.C. Movement Primitives, Principal Component Analysis, and the Efficient Generation of Natural Motions. In Proceedings of the Proceedings of the 2005 IEEE International Conference on Robotics and Automation, Barcelona, Spain, 18–22 April 2005; pp. 4630–4635. [Google Scholar] [CrossRef]
- St-Onge, N.; Feldman, A.G. Interjoint coordination in lower limbs during different movements in humans. Exp. Brain Res. 2003, 148, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Borghese, N.A.; Bianchi, L.; Lacquaniti, F. Kinematic determinants of human locomotion. J. Physiol. 1996, 494, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.D.; Hulliger, M.; Lee, R.G.; O’Callaghan, I.S. Quantitative Analysis of Human Movement Synergies: Constructive Pattern Analysis for Gait. J. Mot. Behav. 1994, 26, 83–102. [Google Scholar] [CrossRef]
- Funato, T.; Aoi, S.; Oshima, H.; Tsuchiya, K. Variant and invariant patterns embedded in human locomotion through whole body kinematic coordination. Exp. Brain Res. 2010, 205, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Hotelling, H. New Light on the Correlation Coefficient and its Transforms. J. R. Stat. Soc. Ser. (Methodol.) 1953, 15, 193–225. [Google Scholar] [CrossRef]
- Vlutters, M.; Van Asseldonk, E.; Van der Kooij, H. Center of mass velocity based predictions in balance recovery following pelvis perturbations during human walking. J. Exp. Biol. 2016, 219, 1514–1523. [Google Scholar] [CrossRef]
- Wang, Y.; Srinivasan, M. Stepping in the direction of the fall: The next foot placement can be predicted from current upper body state in steady-state walking. Biol. Lett. 2014, 10, 20140405. [Google Scholar] [CrossRef] [PubMed]
- Crenna, F.; Rossi, G.B.; Palazzo, A. Instantaneous centre of rotation in human motion: Measurement and computational issues. J. Phys. Conf. Ser. 2016, 772, 012027. [Google Scholar] [CrossRef]
- Scanlan, S.F.; Favre, J.; Andriacchi, T.P. The relationship between peak knee extension at heel-strike of walking and the location of thickest femoral cartilage in ACL reconstructed and healthy contralateral knees. J. Biomech. 2013, 46, 849–854. [Google Scholar] [CrossRef]
- Whittle, M.W. Clinical gait analysis: A review. Hum. Mov. Sci. 1996, 15, 369–387. [Google Scholar] [CrossRef]
- Kharb, A.; Saini, V.; Jain, Y.; Dhiman, S. A review of gait cycle and its parameters. Int. J. Comput. Eng. Manag. 2011, 13, 78–83. [Google Scholar]
- Mills, P.M.; Barrett, R.S. Swing phase mechanics of healthy young and elderly men. Hum. Mov. Sci. 2001, 20, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.W. A new measure of trip risk integrating minimum foot clearance and dynamic stability across the swing phase of gait. J. Biomech. 2017, 55, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ullauri, J.B.; Akiyama, Y.; Okamoto, S.; Yamada, Y. Technique to reduce the minimum toe clearance of young adults during walking to simulate the risk of tripping of the elderly. PLoS ONE 2019, 14, e0217336. [Google Scholar] [CrossRef] [PubMed]
- Mavor, M.P.; Ross, G.B.; Clouthier, A.L.; Karakolis, T.; Graham, R.B. Validation of an IMU Suit for Military-Based Tasks. Sensors 2020, 20, 4280. [Google Scholar] [CrossRef] [PubMed]
- Antunes, R.; Jacob, P.; Meyer, A.; Conditt, M.A.; Roche, M.W.; Verstraete, M.A. Accuracy of Measuring Knee Flexion after TKA through Wearable IMU Sensors. J. Funct. Morphol. Kinesiol. 2021, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Drapeaux, A.; Carlson, K. A Comparison of Inertial Motion Capture Systems: DorsaVi and Xsens. Int. J. Kinesiol. Sport. Sci. 2020, 8, 24. [Google Scholar] [CrossRef]
- Teufl, W.; Lorenz, M.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Towards Inertial Sensor Based Mobile Gait Analysis: Event-Detection and Spatio-Temporal Parameters. Sensors 2019, 19, 38. [Google Scholar] [CrossRef]
- Schwesig, R.; Leuchte, S.; Fischer, D.; Ullmann, R.; Kluttig, A. Inertial sensor based reference gait data for healthy subjects. Gait Posture 2011, 33, 673–678. [Google Scholar] [CrossRef]
- Ciklacandir, S.; Ozkan, S.; Isler, Y. A Comparison of the Performances of Video-Based and IMU Sensor-Based Motion Capture Systems on Joint Angles. In Proceedings of the 2022 Innovations in Intelligent Systems and Applications Conference (ASYU), Antalya, Turkey, 7–9 September 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Cockcroft, J.; Louw, Q.; Baker, R. Proximal placement of lateral thigh skin markers reduces soft tissue artefact during normal gait using the Conventional Gait Model. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 1497–1504. [Google Scholar] [CrossRef]
- WS, E. Center of mass of the human body helps in analysis of balance and movement. MOJ Appl. Bionics Biomech. 2018, 2, 144–148. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Okamoto, S.; Kuroda, T.; Akiyama, Y. Estimating the Margin of Gait Stability in Healthy Elderly Using the Triaxial Kinematic Motion of a Single Body Feature. Appl. Sci. 2024, 14, 3067. https://doi.org/10.3390/app14073067
Liu Z, Okamoto S, Kuroda T, Akiyama Y. Estimating the Margin of Gait Stability in Healthy Elderly Using the Triaxial Kinematic Motion of a Single Body Feature. Applied Sciences. 2024; 14(7):3067. https://doi.org/10.3390/app14073067
Chicago/Turabian StyleLiu, Ziqi, Shogo Okamoto, Tomohito Kuroda, and Yasuhiro Akiyama. 2024. "Estimating the Margin of Gait Stability in Healthy Elderly Using the Triaxial Kinematic Motion of a Single Body Feature" Applied Sciences 14, no. 7: 3067. https://doi.org/10.3390/app14073067
APA StyleLiu, Z., Okamoto, S., Kuroda, T., & Akiyama, Y. (2024). Estimating the Margin of Gait Stability in Healthy Elderly Using the Triaxial Kinematic Motion of a Single Body Feature. Applied Sciences, 14(7), 3067. https://doi.org/10.3390/app14073067




