Thermal Effect of Probes Present in a Pharmaceutical Formulation during Freeze-Drying Measured by Contact-Free Infrared Thermography
Abstract
:1. Introduction
- Annealing of ice to achieve crystals of larger and uniform size [18].
- Sublimation (primary drying), whereby the water evaporates from solid ice in the material and is captured on the ice-condenser under soft vacuum.
- Secondary drying during which most of the remaining water is removed under hard vacuum and a shelf temperature of 20 to 30 °C.
2. Materials and Methods
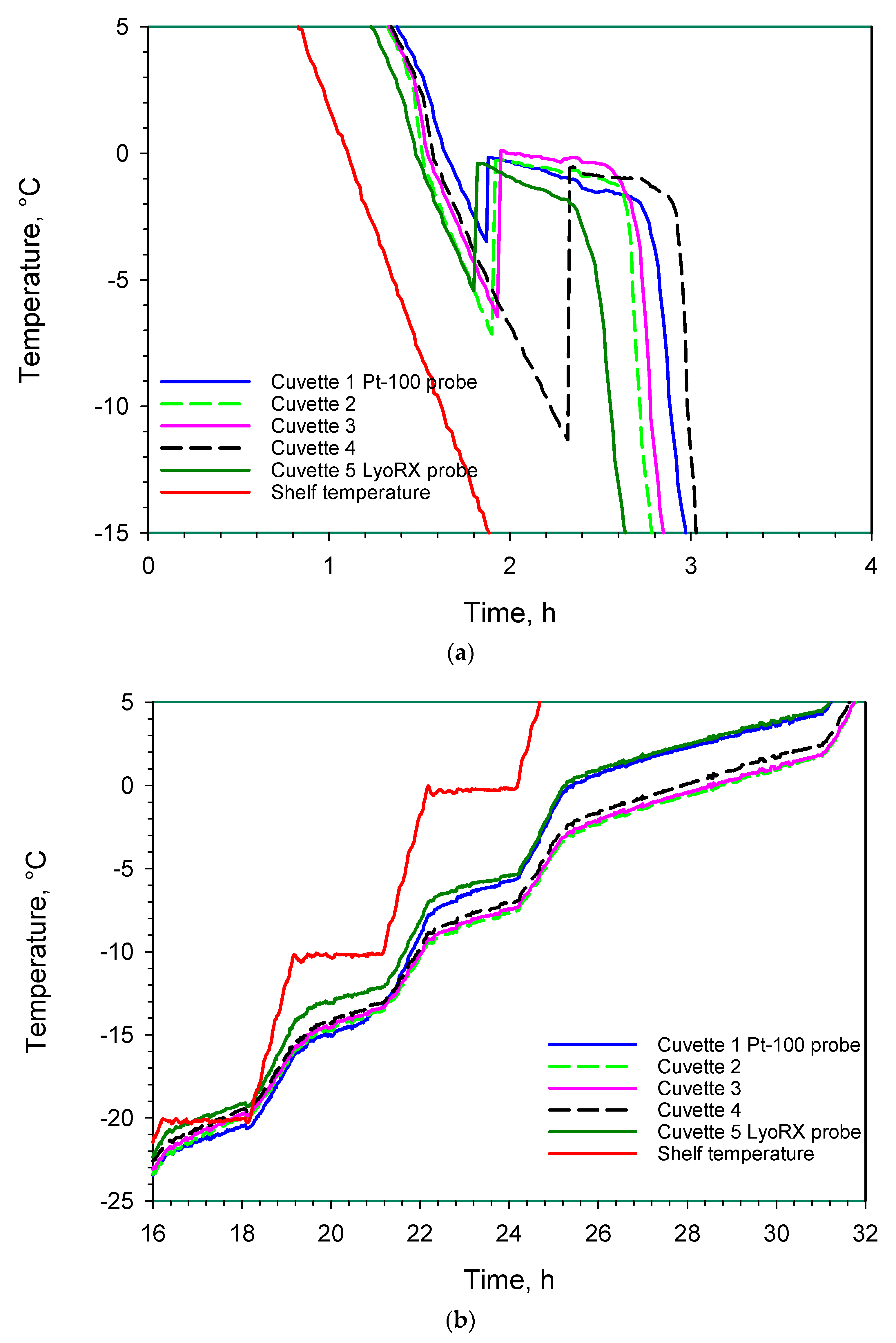
3. Results and Discussion
3.1. Monitoring of A Freeze-Drying Cycle for a Mannitol/Sucrose Solution
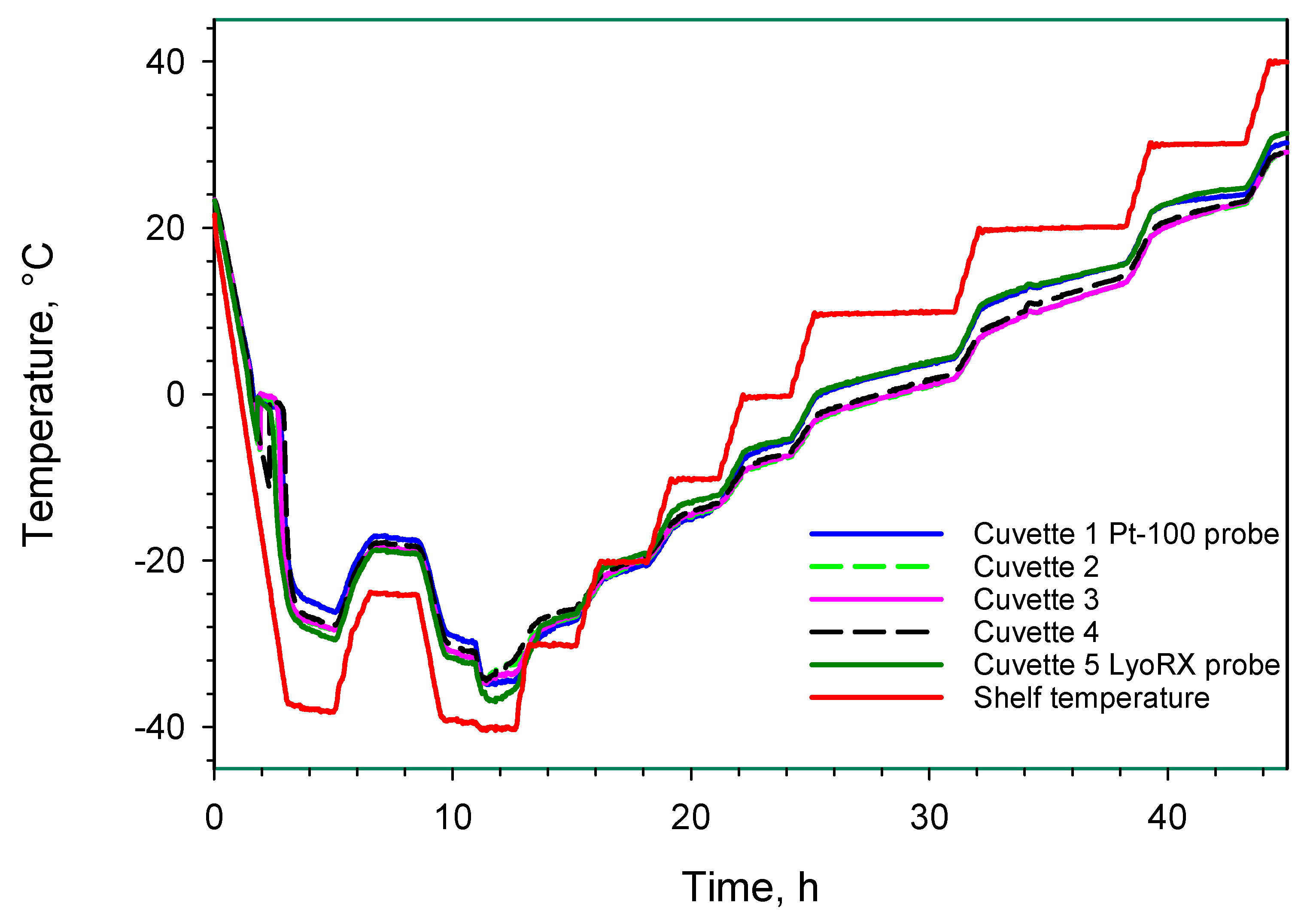
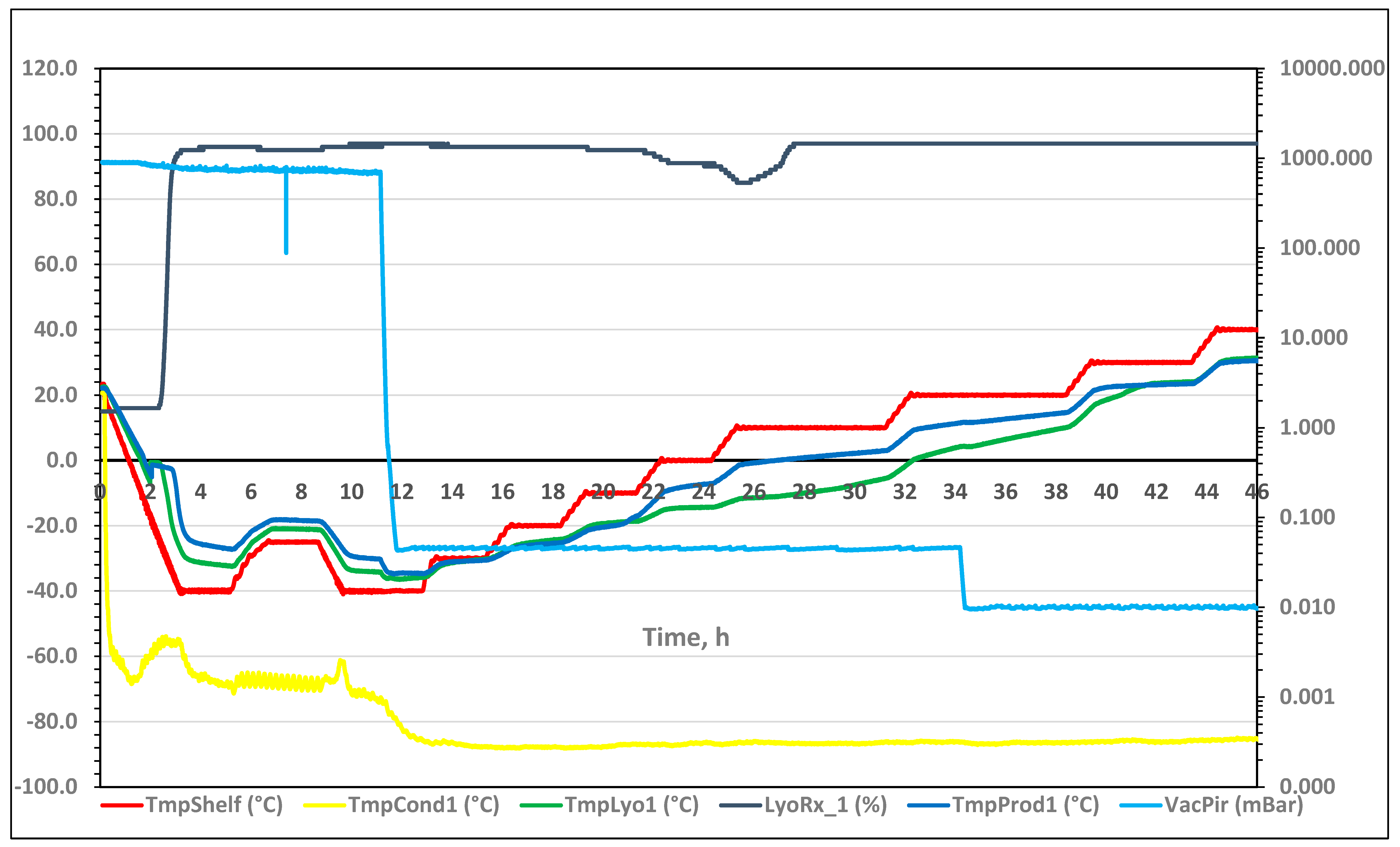
3.1.1. Full Freeze Drying Cycle
3.1.2. Supercooling and Freezing
3.1.3. Sublimation/Primary Drying
3.1.4. Secondary Drying
3.1.5. Residual Water Content in the Freeze-Dried Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaim
References
- Ohtake, S.; Izutsu, K.; Lechuga Ballesteros, D. (Eds.) Drying Technologies for Biotechnology and Pharmaceutical Applications; Wiley-VCH: Weinheim, Germany, 2020; ISBN 9783527341122. [Google Scholar]
- Difranco, N. Lyophilization of Pharmaceuticals: An Overview. Lubrizol CDMO. 8 October 2019. Available online: https://lubrizolcdmo.com/blog/lyophilization-of-pharmaceuticals-an-overview/ (accessed on 29 February 2024).
- Available online: https://en.wikipedia.org/wiki/Resistance_thermometer#Pt (accessed on 29 February 2024).
- Roy, M.L.; Pikal, M.J. Process control in freeze-drying: Determination of the end point of sublimation drying by an electronic moisture sensor. J. Parenter. Sci. Technol. 1989, 43, 60–66. [Google Scholar]
- Emteborg, H.; Zeleny, R.; Charoud-Got, J.; Martos, G.; Lüddeke, J.; Schellin, H.; Teipel, K. Infrared Thermography for Monitoring of Freeze-Drying Processes: Instrumental Developments and Preliminary Results. J. Pharm. Sciences 2014, 103, 2088–2097. [Google Scholar] [CrossRef]
- Emteborg, H.; Charoud-Got, J.; Seghers, J. Infrared Thermography for Monitoring of Freeze Drying Processes—Part 2: Monitoring of Temperature on the Surface and Vertically in Cuvettes during Freeze Drying of a Pharmaceutical Formulation. Pharmaceutics 2022, 14, 1007. [Google Scholar] [CrossRef]
- Fissore, D.; Pisano, R.; Barres, A.A. Process analytical technology for monitoring pharmaceuticals freeze-drying–A comprehensive review. Dry. Technol. 2018, 36, 1839–1865. [Google Scholar] [CrossRef]
- Van Bockstal, P.J.; Corver, J.; De Meyer, L.; Vervaet, C.; De Beer, T. Thermal Imaging as a Noncontact Inline Process Analytical Tool for Product Temperature Monitoring during Continuous Freeze-Drying of Unit Doses. Anal. Chem. 2018, 90, 13591–13599. [Google Scholar] [CrossRef] [PubMed]
- Lietta, E.; Colucci, D.; Distefano, G.; Fissore, D. On the Use of Infrared Thermography for Monitoring a Vial Freeze-Drying Process. J. Pharm. Sci. 2019, 108, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Colucci, D.; Prats-Montalbán, J.M.; Fissore, D.; Ferrer, A. Application of multivariate image analysis for on-line monitoring of a freeze-drying process for pharmaceutical products in vials. Chemom. Intell. Lab. Syst. 2019, 187, 19–27. [Google Scholar] [CrossRef]
- Colucci, D.; Maniaci, R.; Fissore, D. Monitoring of the freezing stage in a freeze-drying process using IR thermography. Int. J. Pharm. 2019, 566, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Klijn, M.E.; Hubbuch, J. Application of ultraviolet, visible, and infrared light imaging in protein-based biopharmaceutical formulation characterization and development studies. Eur. J. Pharm. Biopharm. 2021, 165, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Harguindeguy, M.; Stratta, L.; Fissore, D.; Pisano, R. Investigation of the freezing phenomenon in vials using an infrared camera. Pharmaceutics 2021, 13, 1664. [Google Scholar] [CrossRef] [PubMed]
- Vallan, A.; Fissore, D.; Pisano, R.; Barresi, A.A. On the Use of Temperature Measurements as a Process Analytical Technology (PAT) for the Monitoring of a Pharmaceutical Freeze-Drying Process. Pharmaceutics 2023, 15, 861. [Google Scholar] [CrossRef]
- Jiang, X.; Kazarin, P.; Sinanis, M.D.; Darwish, A.; Raghunathan, N.; Alexeenko, A.; Peroulis, D. A non-invasive multipoint product temperature measurement for pharmaceutical lyophilisation. Sci. Rep. 2022, 12, 12010. [Google Scholar] [CrossRef] [PubMed]
- Juckers, A.; Knerr, P.; Harms, F.; Strube, J. Effect of the Freezing Step on Primary Drying Experiments and Simulation of Lyophilization Processes. Processes 2023, 11, 1404. [Google Scholar] [CrossRef]
- Geidobler, R.; Winter, G. Controlled ice nucleation in the field of freeze-drying: Fundamentals and technology review. Eur. J. Pharm. Biopharm. 2013, 85, 214–222. [Google Scholar] [CrossRef]
- Hawe, A.; Friess, W. Impact of freezing procedure and annealing on the physico-chemical properties and the formation of mannitol hydrate in mannitol-sucrose-NaCl formulations. Eur. J. Pharm. Biopharm. 2006, 64, 316–325. [Google Scholar] [CrossRef]
- Available online: https://www.flir.eu/products/flir-one-pro/ (accessed on 29 February 2024).
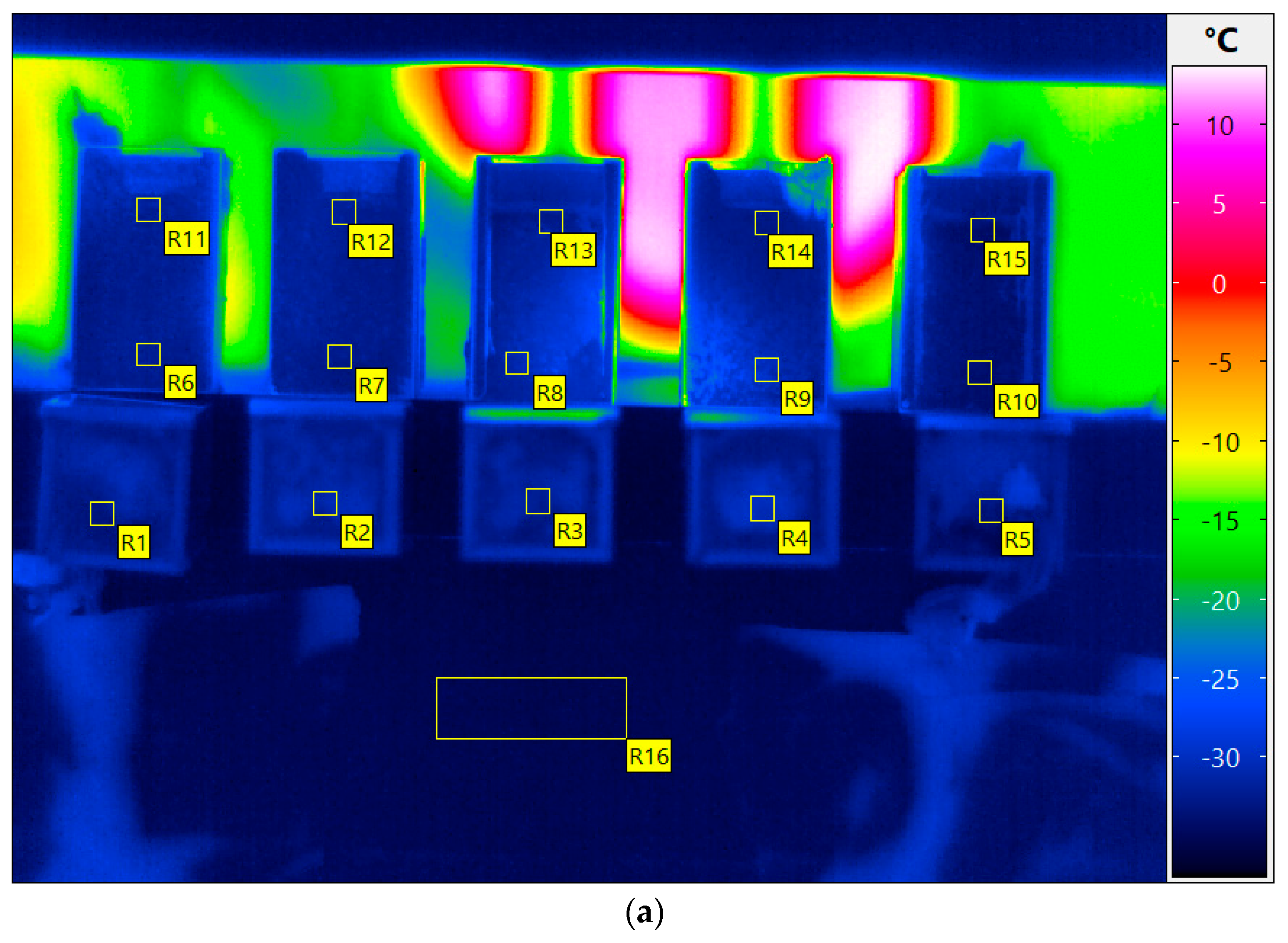
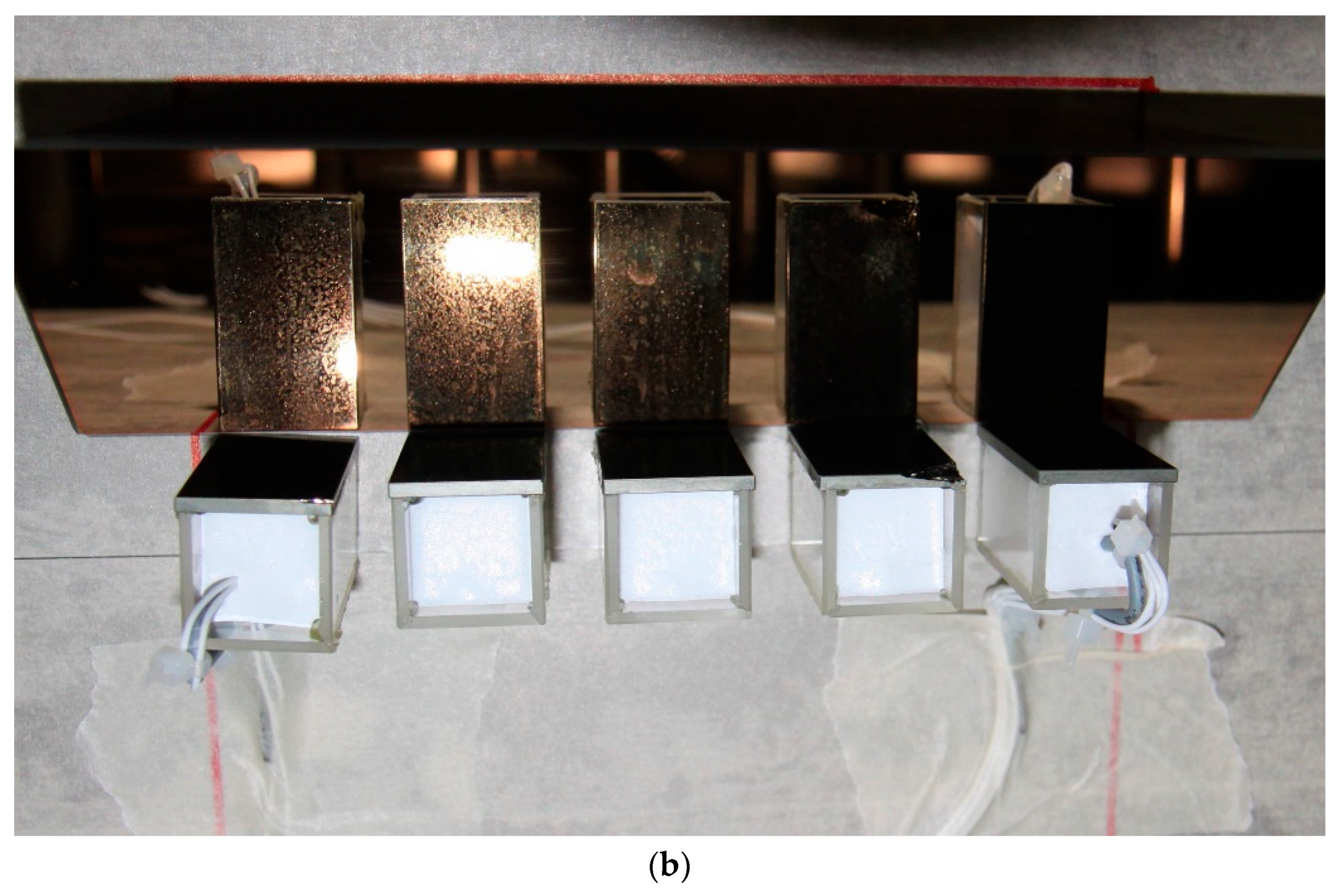
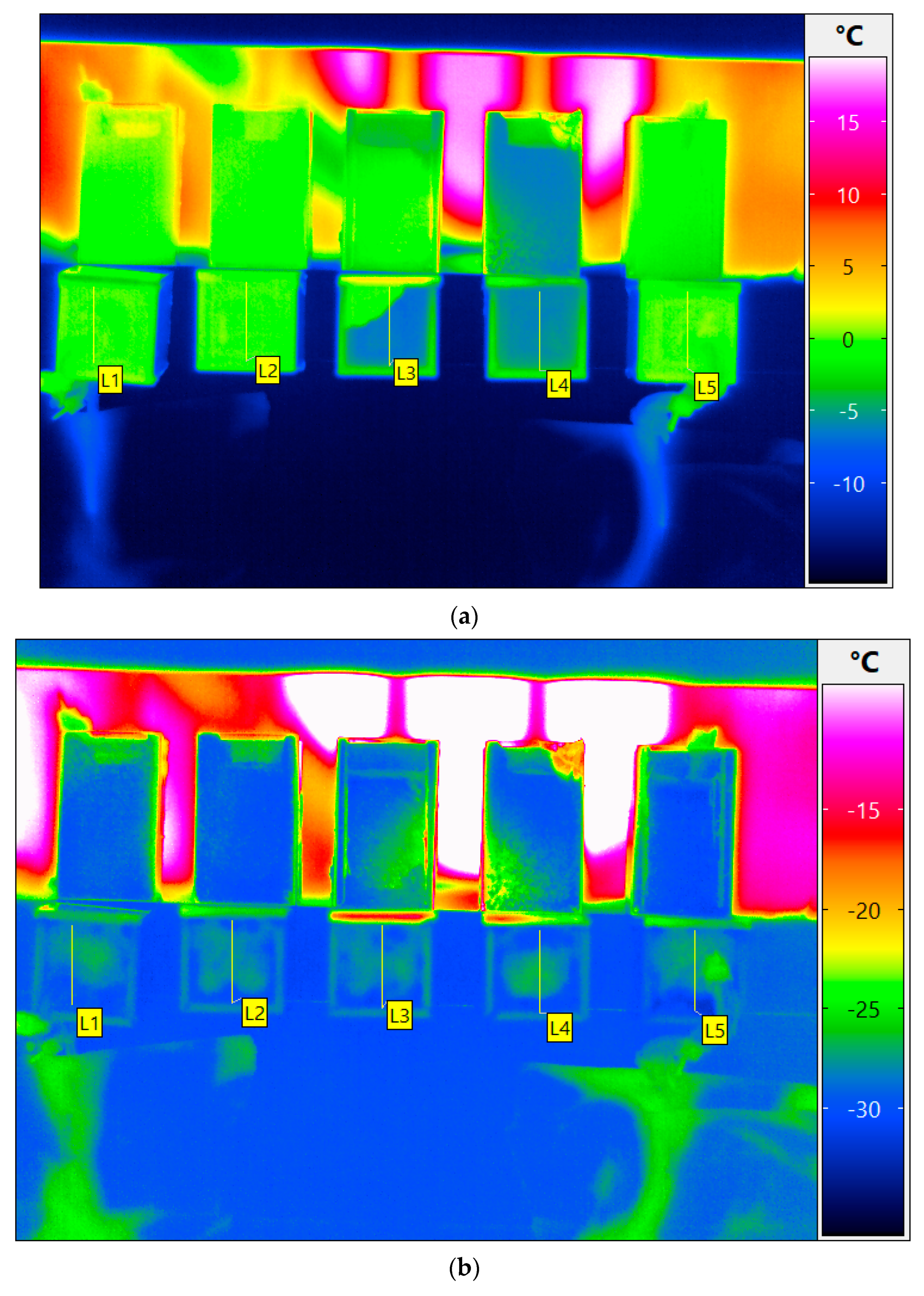
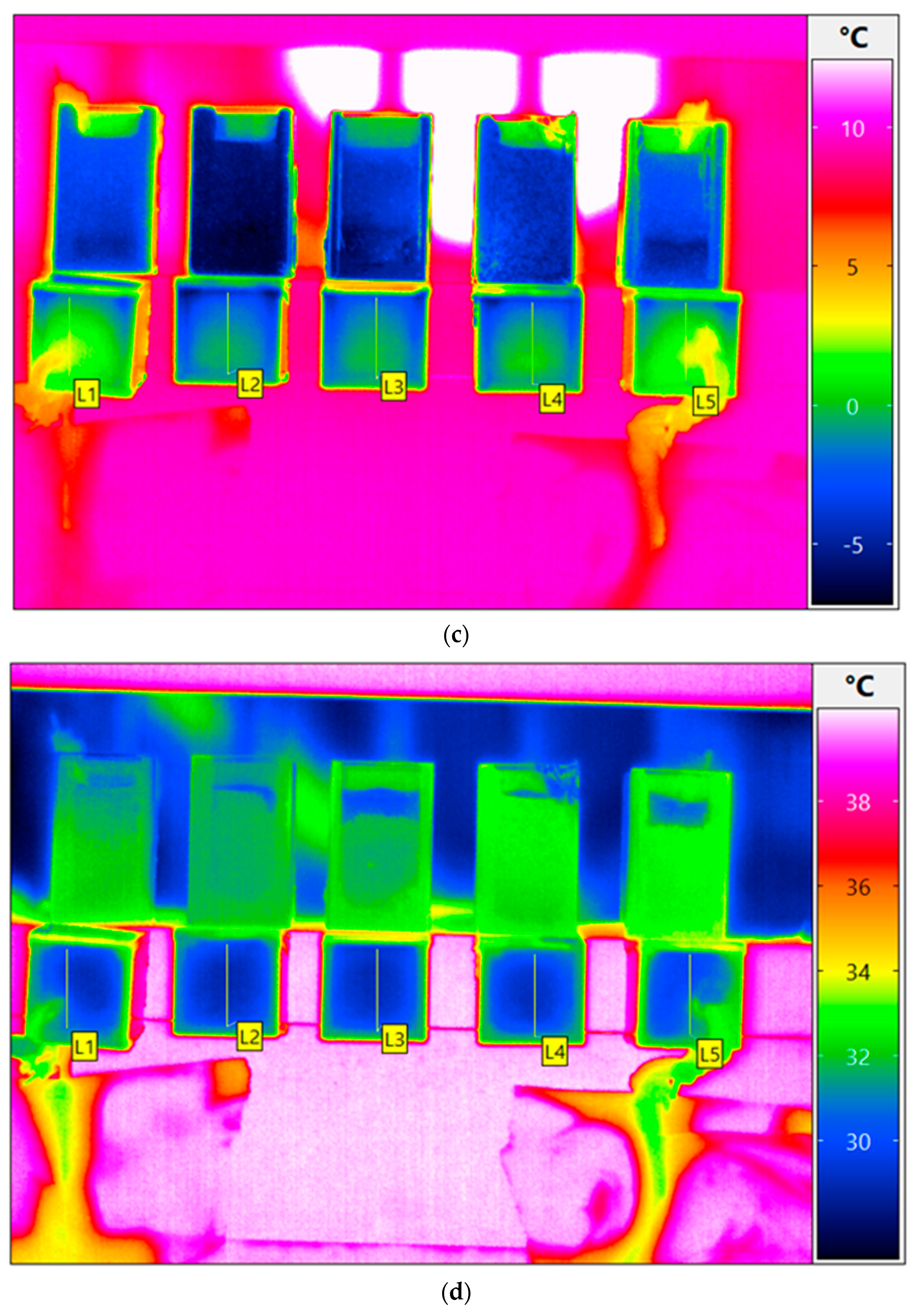
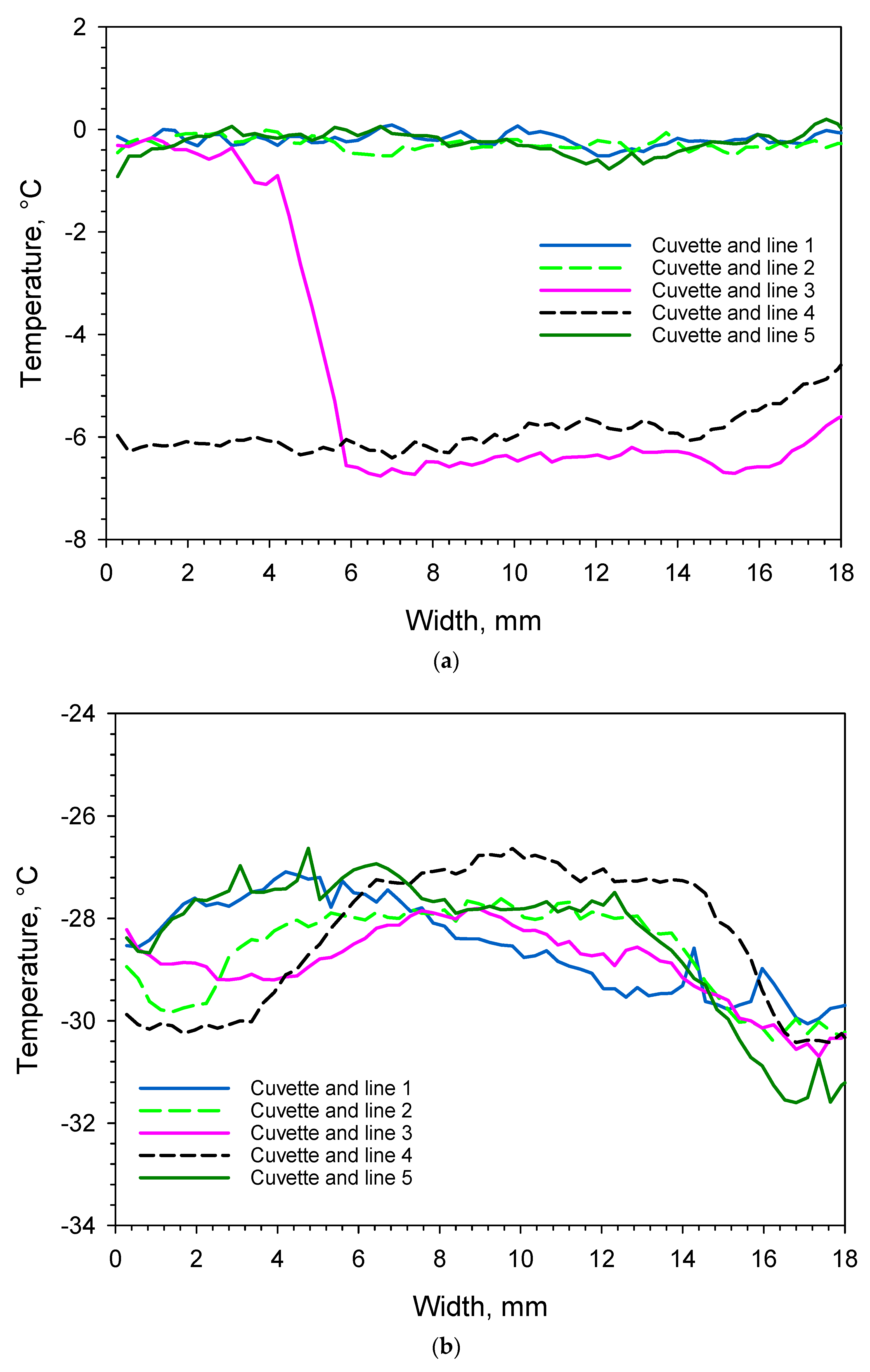
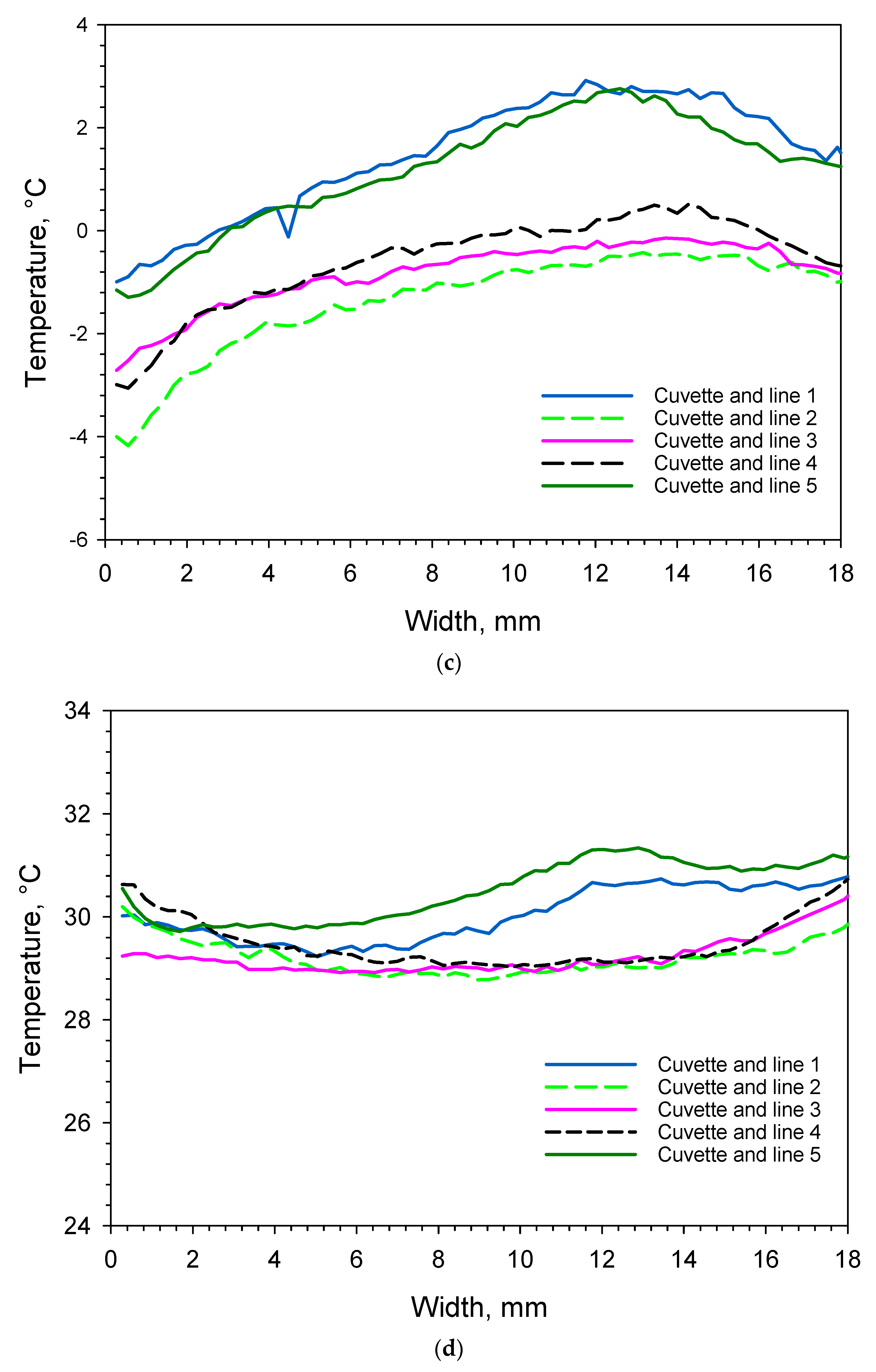
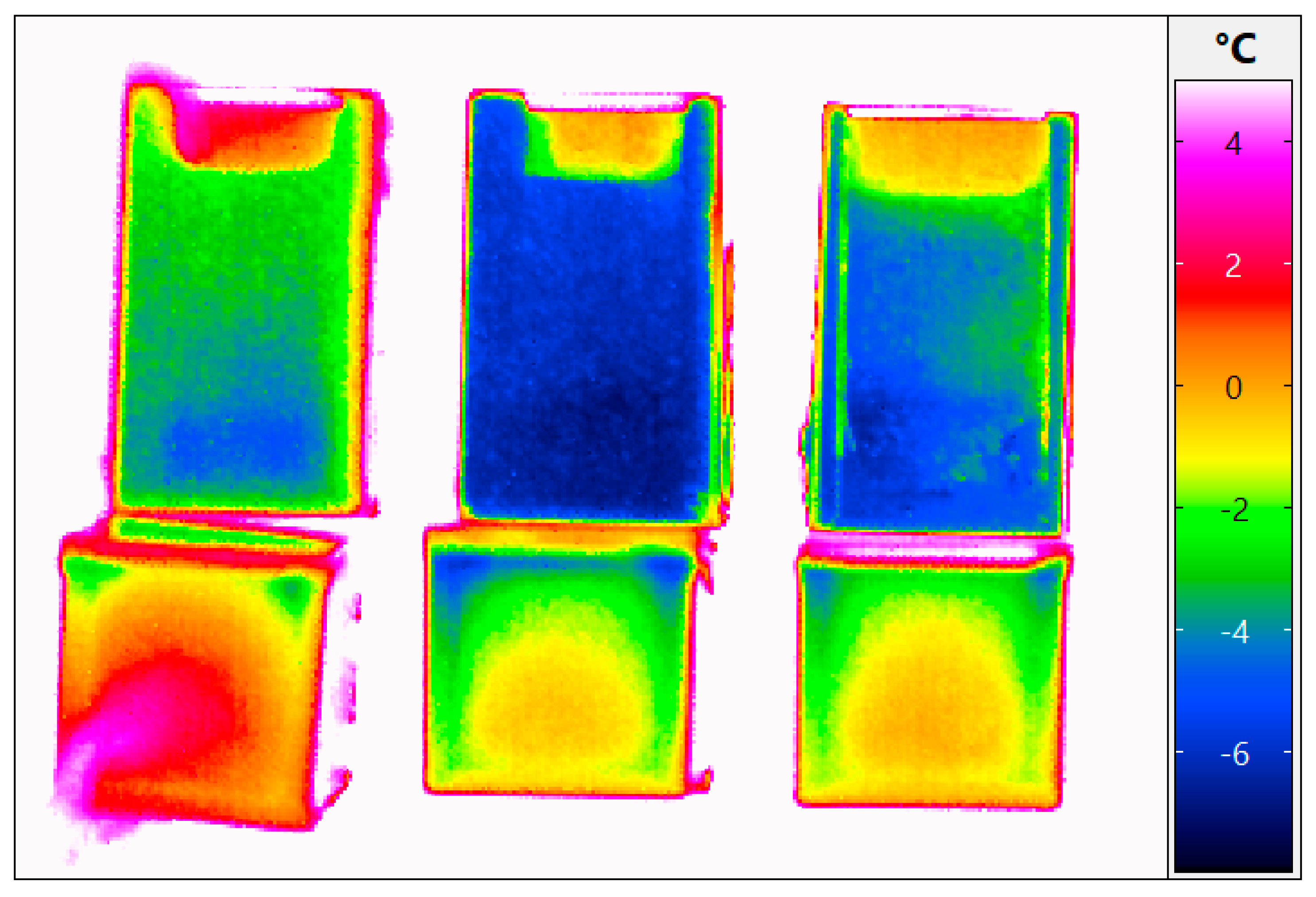
| Cuvette Number as Given in Figure 1a | Water Content, % (m/m) | Order of Freezing | Temperature of Super-Cooled Liquid Before Nucleation and Freezing, °C |
|---|---|---|---|
| 1 * | 5.81 | 2 | −3.5 |
| 2 | 5.79 | 3 | −7.0 |
| 3 | 6.15 | 4 | −6.5 |
| 4 | 5.95 | 5 | −11.5 |
| 5 * | 5.57 | 1 | −5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emteborg, H.; Charoud-Got, J. Thermal Effect of Probes Present in a Pharmaceutical Formulation during Freeze-Drying Measured by Contact-Free Infrared Thermography. Appl. Sci. 2024, 14, 3120. https://doi.org/10.3390/app14073120
Emteborg H, Charoud-Got J. Thermal Effect of Probes Present in a Pharmaceutical Formulation during Freeze-Drying Measured by Contact-Free Infrared Thermography. Applied Sciences. 2024; 14(7):3120. https://doi.org/10.3390/app14073120
Chicago/Turabian StyleEmteborg, Håkan, and Jean Charoud-Got. 2024. "Thermal Effect of Probes Present in a Pharmaceutical Formulation during Freeze-Drying Measured by Contact-Free Infrared Thermography" Applied Sciences 14, no. 7: 3120. https://doi.org/10.3390/app14073120






