Impact of Prolonged Sitting Interruption on Blood Glucose, Insulin and Triacylglycerol in Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Risk-of-Bias Assessment
2.4. Statistical Analysis
3. Results
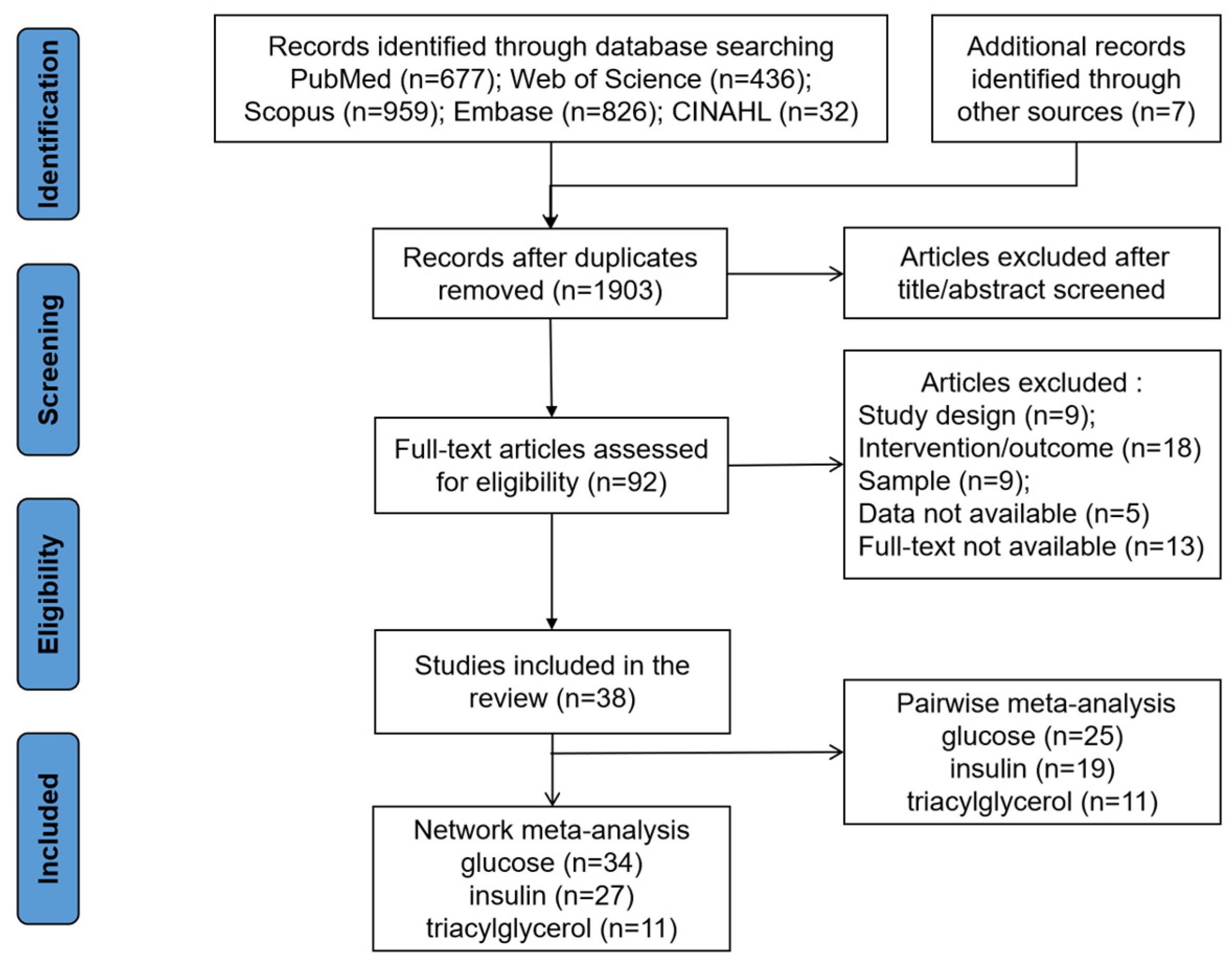
3.1. Literature Search and Study Characteristics
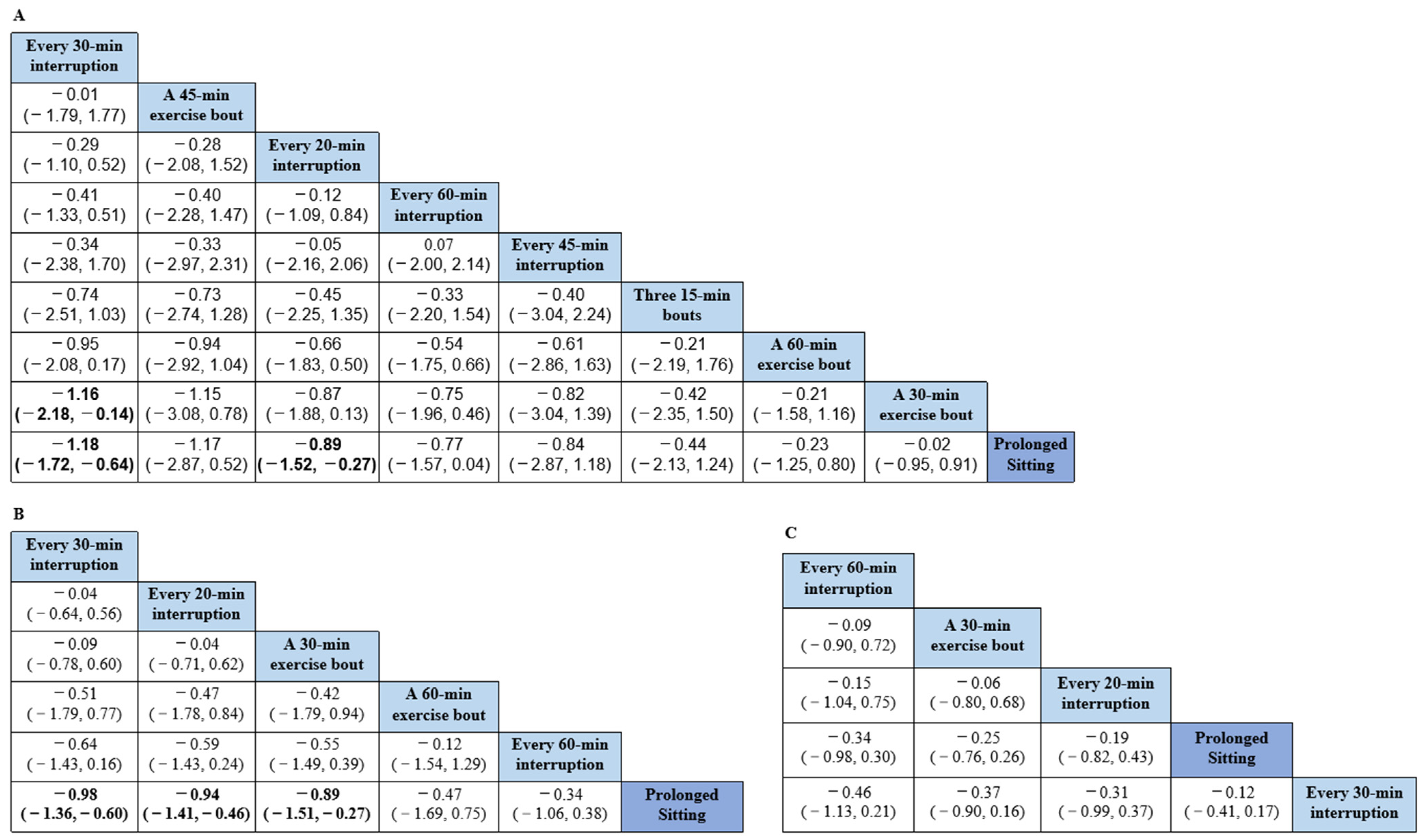
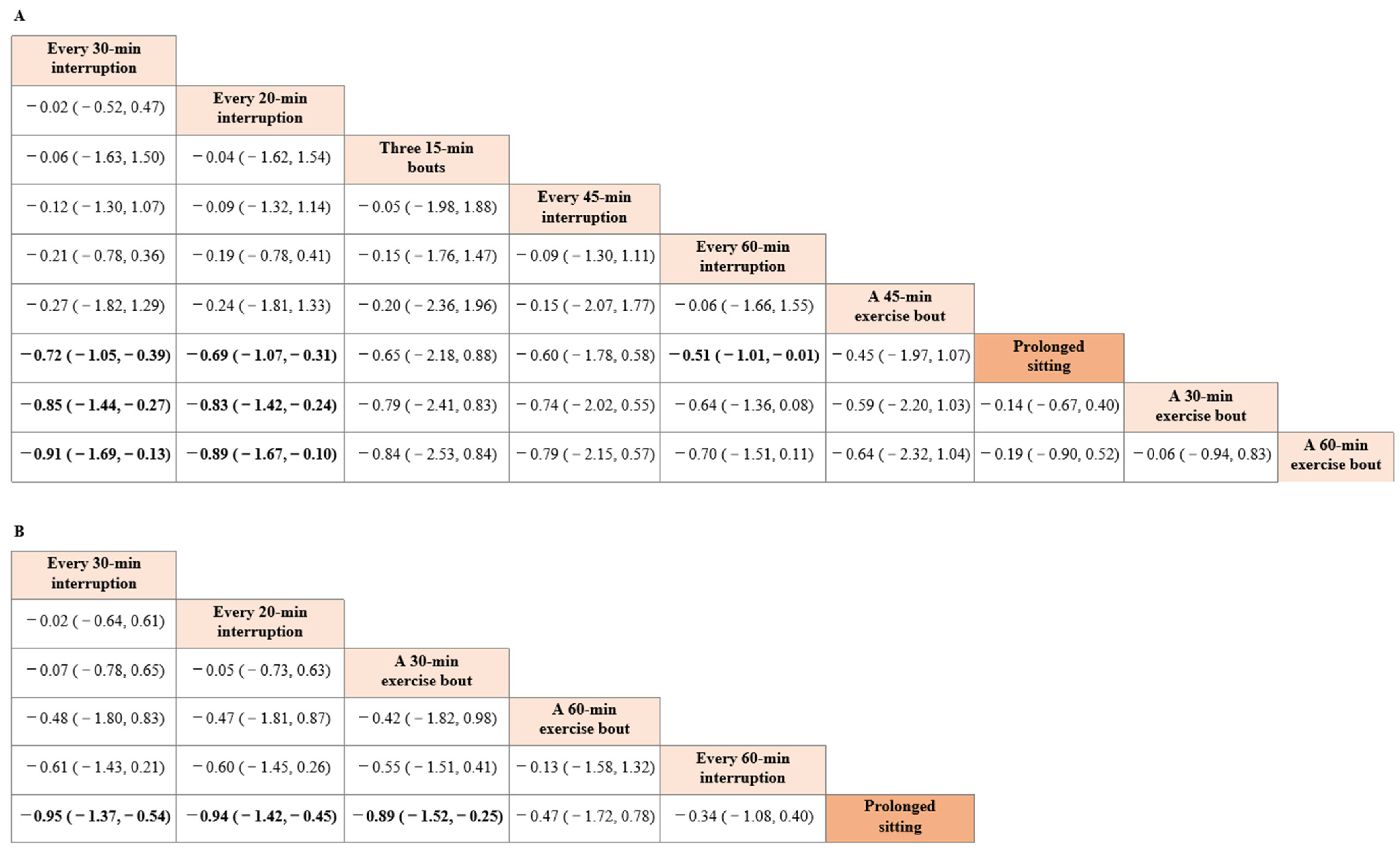
3.2. Network Meta-Analysis
3.3. Pairwise Meta-Analysis
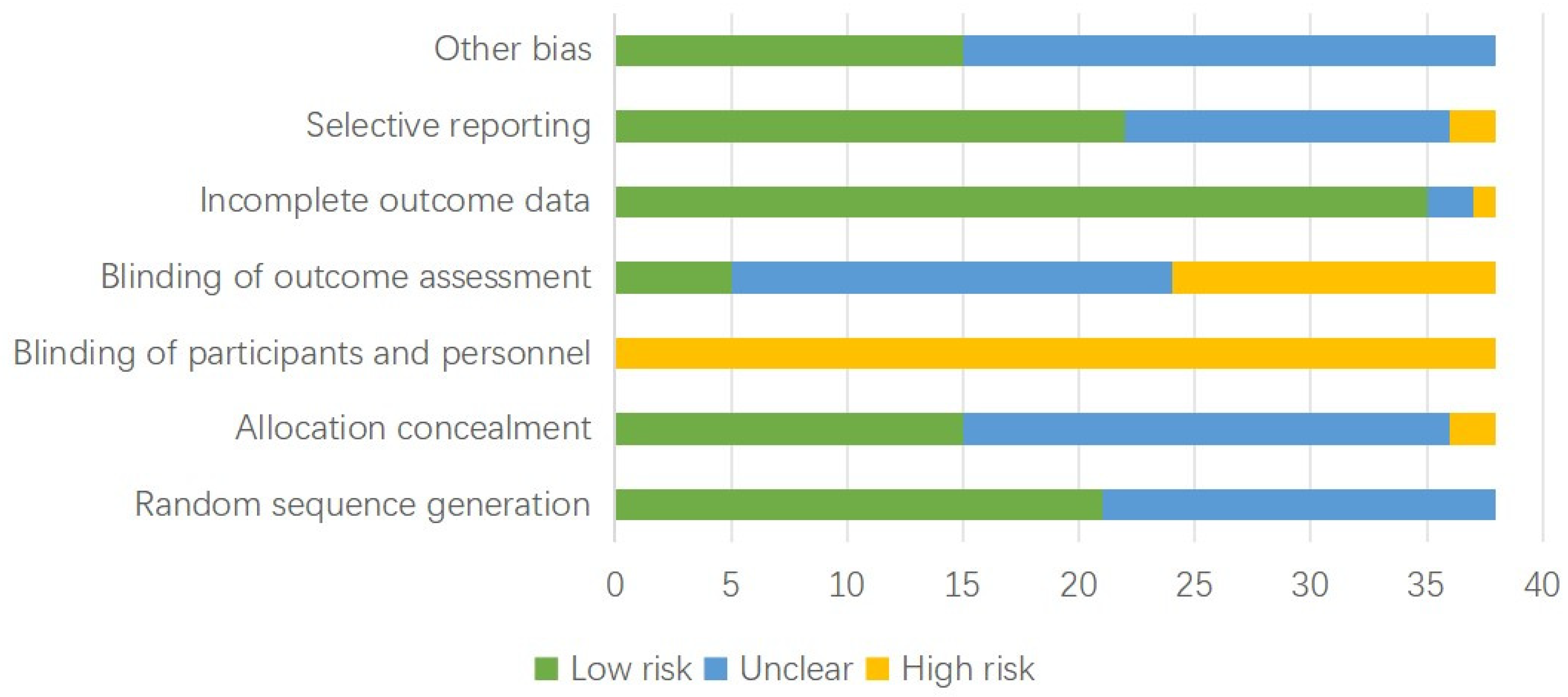
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syeda, U.S.A. The importance of exercise for glycemic control in type 2 diabetes. Am. J. Med. Open 2023, 9, 100031. [Google Scholar] [CrossRef]
- Dempsey, P.C.; Owen, N.; Yates, T.E.; Kingwell, B.A.; Dunstan, D.W. Sitting Less and Moving More: Improved Glycaemic Control for Type 2 Diabetes Prevention and Management. Curr. Diabetes Rep. 2016, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.N.; Kingwell, B.A.; Robinson, C.; Hammond, L.; Cerin, E.; Shaw, J.E.; Healy, G.N.; Hamilton, M.T.; Owen, N.; Dunstan, D.W. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin. Sci. 2015, 129, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Fuezeki, E.; Engeroff, T.; Banzer, W. Health Benefits of Light-Intensity Physical Activity: A Systematic Review of Accelerometer Data of the National Health and Nutrition Examination Survey (NHANES). Sports Med. 2017, 47, 1769–1793. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Della Guardia, L.; Codella, R. Exercise Restores Hypothalamic Health in Obesity by Reshaping the Inflammatory Network. Antioxidants 2023, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Merwin, R.M. The Role of Exercise in Management of Mental Health Disorders: An Integrative Review. Annu. Rev. Med. 2021, 72, 45–62. [Google Scholar] [CrossRef]
- Loh, R.; Stamatakis, E.; Folkerts, D.; Allgrove, J.E.; Moir, H.J. Effects of Interrupting Prolonged Sitting with Physical Activity Breaks on Blood Glucose, Insulin and Triacylglycerol Measures: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 295–330. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Kingwell, B.A.; Larsen, R.; Healy, G.N.; Cerin, E.; Hamilton, M.T.; Shaw, J.E.; Bertovic, D.A.; Zimmet, P.Z.; Salmon, J.; et al. Breaking Up Prolonged Sitting Reduces Postprandial Glucose and Insulin Responses. Diabetes Care 2012, 35, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Bhammar, D.M.; Sawyer, B.J.; Tucker, W.J.; Gaesser, G.A. Breaks in Sitting Time: Effects on Continuously Monitored Glucose and Blood Pressure. Med. Sci. Sports Exerc. 2017, 49, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ-Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, M.; Thomsen, C.; Hermansen, K. Incremental area under response curve more accurately describes the triglyceride response to an oral fat load in both healthy and type 2 diabetic subjects. Metab. Clin. Exp. 2003, 52, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br. J. Nutr. 2004, 91, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Caldwell, D.M.; Lu, G.; Ades, A.E. Evidence Synthesis for Decision Making 4: Inconsistency in Networks of Evidence Based on Randomized Controlled Trials. Med. Decis. Mak. 2013, 33, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Vasiliadis, H.S.; Higgins, J.P.; Salanti, G. Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 2013, 42, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Terrin, N.; Schmid, C.H.; Lau, J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J. Clin. Epidemiol. 2005, 58, 894–901. [Google Scholar] [CrossRef]
- Bailey, D.P.; Stringer, C.A.; Maylor, B.D.; Zakrzewski-Fruer, J.K. Lower Amounts of Daily and Prolonged Sitting Do Not Lower Free-Living Continuously Monitored Glucose Concentrations in Overweight and Obese Adults: A Randomised Crossover Study. Nutrients 2022, 14, 605. [Google Scholar] [CrossRef]
- Blankenship, J.M.; Granados, K.; Braun, B. Effects of subtracting sitting versus adding exercise on glycemic control and variability in sedentary office workers. Appl. Physiol. Nutr. Metab. 2014, 39, 1286–1293. [Google Scholar] [CrossRef]
- Chrismas, B.C.R.; Taylor, L.; Cherif, A.; Sayegh, S.; Rizk, N.; El-Gamal, A.; Allenjawi, S.H.; Bailey, D.P. Postprandial Insulin and Triglyceride Concentrations Are Suppressed in Response to Breaking Up Prolonged Sitting in Qatari Females. Front. Physiol. 2019, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Duvivier, B.M.F.M.; Schaper, N.C.; Koster, A.; van Kan, L.; Peters, H.P.F.; Adam, J.J.; Giesbrecht, T.; Kornips, E.; Hulsbosch, M.; Willems, P.; et al. Benefits of Substitution Sitting with Standing and Walking in Free-living Conditions for Cardiometabolic Risk Markers, cognition and Mood in Overweight Adults. Front. Physiol. 2017, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Hawari, N.S.A.; Wilson, J.; Gill, J.M.R. Effects of breaking up sedentary time with “chair squats” on postprandial metabolism. J. Sports Sci. 2019, 37, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Davies, M.J.; Bodicoat, D.H.; Edwardson, C.L.; Gill, J.M.R.; Stensel, D.J.; Tolfrey, K.; Dunstan, D.W.; Khunti, K.; Yates, T. Breaking Up Prolonged Sitting With Standing or Walking Attenuates the Postprandial Metabolic Response in Postmenopausal Women: A Randomized Acute Study. Diabetes Care 2016, 39, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, M.; Fairchild, T.; Keslacy, S.; Weinstock, R.; Kanaley, J. Multiple short bouts of exercise over 12-h period reduce glucose excursions more than an energy-matched single bout of exercise. Metab.-Clin. Exp. 2014, 63, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Crist, K.; Vital, D.G.; Dillon, L.; Aden, S.A.; Trivedi, M.; Castellanos, L.R.; Godbole, S.; Li, H.; Allison, M.A.; et al. Acute glucoregulatory and vascular outcomes of three strategies for interrupting prolonged sitting time in postmenopausal women: A pilot, laboratory-based, randomized, controlled, 4-condition, 4-period crossover trial. PLoS ONE 2017, 12, e0188544. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Edwardson, C.L.; Davies, M.J.; Henson, J.; Rowlands, A.; King, J.A.; Bodicoat, D.H.; Khunti, K.; Yates, T. Breaking up sedentary time with seated upper body activity can regulate metabolic health in obese high-risk adults: A randomized crossover trial. Diabetes Obes. Metab. 2017, 19, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Newsom, S.A.; Everett, A.C.; Hinko, A.; Horowitz, J.F. A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes Care 2013, 36, 2516–2522. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Green, D.J.; Cerin, E.; Ellis, K.A.; Heinonen, I.; Lewis, J.; Naylor, L.H.; Cohen, N.; Larsen, R.; Dempsey, P.C.; et al. Combined effects of continuous exercise and intermittent active interruptions to prolonged sitting on postprandial glucose, insulin, and triglycerides in adults with obesity: A randomized crossover trial. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 152. [Google Scholar] [CrossRef]
- Yates, T.; Edwardson, C.L.; Celis-Morales, C.; Biddle, S.J.H.; Bodicoat, D.; Davies, M.J.; Esliger, D.; Henson, J.; Kazi, A.; Khunti, K.; et al. Metabolic Effects of Breaking Prolonged Sitting With Standing or Light Walking in Older South Asians and White Europeans: A Randomized Acute Study. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2020, 75, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Betts, J.A.; Walhin, J.P.; Thompson, D. Adipose Tissue Responses to Breaking Sitting in Men and Women with Central Adiposity. Med. Sci. Sports Exerc. 2018, 50, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Wongpipit, W.; Huang, W.Y.; Miyashita, M.; Tian, X.Y.; Wong, S.H.-S. Frequency of interruptions to prolonged sitting and postprandial metabolic responses in young, obese, Chinese men. J. Sports Sci. 2021, 39, 1376–1385. [Google Scholar] [CrossRef]
- Altenburg, T.M.; Rotteveel, J.; Serne, E.H.; Chinapaw, M.J.M. Standing is not enough: A randomized crossover study on the acute cardiometabolic effects of variations in sitting in healthy young men. J. Sci. Med. Sport 2019, 22, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.P.; Broom, D.R.; Chrismas, B.C.R.; Taylor, L.; Flynn, E.; Hough, J. Breaking up prolonged sitting time with walking does not affect appetite or gut hormone concentrations but does induce an energy deficit and suppresses postprandial glycaemia in sedentary adults. Appl. Physiol. Nutr. Metab. 2016, 41, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.P.; Locke, C.D. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J. Sci. Med. Sport 2015, 18, 294–298. [Google Scholar] [CrossRef]
- Charlett, O.P.; Morari, V.; Bailey, D.P. Impaired postprandial glucose and no improvement in other cardiometabolic responses or cognitive function by breaking up sitting with bodyweight resistance exercises: A randomised crossover trial. J. Sports Sci. 2021, 39, 792–800. [Google Scholar] [CrossRef]
- Duvivier, B.M.F.M.; Schaper, N.C.; Bremers, M.A.; van Crombrugge, G.; Menheere, P.P.C.A.; Kars, M.; Savelberg, H.H.C.M. Minimal Intensity Physical Activity (Standing and Walking) of Longer Duration Improves Insulin Action and Plasma Lipids More than Shorter Periods of Moderate to Vigorous Exercise (Cycling) in Sedentary Subjects When Energy Expenditure Is Comparable. PLoS ONE 2013, 8, e55542. [Google Scholar] [CrossRef]
- Hansen, R.K.; Andersen, J.B.; Vinther, A.S.; Pielmeier, U.; Larsen, R.G. Breaking up Prolonged Sitting does not Alter Postprandial Glycemia in Young, Normal-Weight Men and Women. Int. J. Sports Med. 2016, 37, 1097–1102. [Google Scholar] [CrossRef]
- McCarthy, M.; Edwardson, C.L.; Davies, M.J.; Henson, J.; Bodicoat, D.H.; Khunti, K.; Dunstan, D.W.; King, J.A.; Yates, T. Fitness Moderates Glycemic Responses to Sitting and Light Activity Breaks. Med. Sci. Sports Exerc. 2017, 49, 2216–2222. [Google Scholar] [CrossRef]
- Peddie, M.C.; Bone, J.L.; Rehrer, N.J.; Skeaff, C.M.; Gray, A.R.; Perry, T.L. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: A randomized crossover trial. Am. J. Clin. Nutr. 2013, 98, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Peddie, M.C.; Kessell, C.; Bergen, T.; Gibbons, T.D.; Campbell, H.A.; Cotter, J.D.; Rehrer, N.J.; Thomas, K.N. The effects of prolonged sitting, prolonged standing, and activity breaks on vascular function, and postprandial glucose and insulin responses: A randomised crossover trial. PLoS ONE 2021, 16, e0244841. [Google Scholar] [CrossRef] [PubMed]
- Benatti, F.B.; Larsen, S.A.; Kofoed, K.; Nielsen, S.T.; Harder-Lauridsen, N.M.; LyngbÆk, M.P.; Eriksen, D.; Karstoft, K.; Krogh-Madsen, R.; Pedersen, B.K.; et al. Intermittent Standing but not a Moderate Exercise Bout Reduces Postprandial Glycemia. Med. Sci. Sports Exerc. 2017, 49, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Champion, R.B.; Smith, L.R.; Smith, J.; Hirlav, B.; Maylor, B.D.; White, S.L.; Bailey, D.P. Reducing prolonged sedentary time using a treadmill desk acutely improves cardiometabolic risk markers in male and female adults. J. Sports Sci. 2018, 36, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, L.; Gribok, A.; Stevens, M.S.; Hamm, L.F.; Rumpler, W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care 2013, 36, 3262–3268. [Google Scholar] [CrossRef] [PubMed]
- Maylor, B.D.; Zakrzewski-Fruer, J.K.; Stensel, D.J.; Orton, C.J.; Bailey, D.P. Effects of Frequency and Duration of Interrupting Sitting on Cardiometabolic Risk Markers. Int. J. Sports Med. 2019, 40, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Pulsford, R.; Blackwell, J.; Hillsdon, M.; Kos, K. Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: A randomised cross-over study in inactive middle-aged men. J. Sci. Med. Sport 2017, 20, 278–283. [Google Scholar] [CrossRef]
- Dempsey, P.C.; Sacre, J.W.; Larsen, R.N.; Straznicky, N.E.; Sethi, P.; Cohen, N.D.; Cerin, E.; Lambert, G.W.; Owen, N.; Kingwell, B.A.; et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J. Hypertens. 2016, 34, 2376–2382. [Google Scholar] [CrossRef]
- Duvivier, B.M.F.M.; Schaper, N.C.; Hesselink, M.K.C.; van Kan, L.; Stienen, N.; Winkens, B.; Koster, A.; Savelberg, H.H.C.M. Breaking sitting with light activities vs structured exercise: A randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia 2017, 60, 490–498. [Google Scholar] [CrossRef]
- Honda, H.; Igaki, M.; Hatanaka, Y.; Komatsu, M.; Tanaka, S.-I.; Miki, T.; Suzuki, T.; Takaishi, T.; Hayashi, T. Stair climbing/descending exercise for a short time decreases blood glucose levels after a meal in people with type 2 diabetes. BMJ Open Diabetes Res. Care 2016, 4, e000232. [Google Scholar] [CrossRef]
- van Dijk, J.-W.; Venema, M.; van Mechelen, W.; Stehouwer, C.D.A.; Hartgens, F.; van Loon, L.J.C. Effect of Moderate-Intensity Exercise versus Activities of Daily Living on 24-Hour Blood Glucose Homeostasis in Male Patients with Type 2 Diabetes. Diabetes Care 2013, 36, 3448–3453. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Edamoto, K.; Kidokoro, T.; Yanaoka, T.; Kashiwabara, K.; Takahashi, M.; Burns, S. Interrupting Sitting Time with Regular Walks Attenuates Postprandial Triglycerides. Int. J. Sports Med. 2016, 37, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kashiwabara, K.; Kidokoro, T.; Yanaoka, T.; Burns, S.F.; Stensel, D.J.; Miyashita, M. Different Patterns of Walking and Postprandial Triglycerides in Older Women. Med. Sci. Sports Exerc. 2018, 50, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-X.; Zhu, Z.; Zhang, L.; Liu, X.-M.; Lin, Y.-Y.; Cao, Z.-B. Metabolic Effects of Three Different Activity Bouts during Sitting in Inactive Adults. Med. Sci. Sports Exerc. 2020, 52, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.C.; Larsen, R.N.; Sethi, P.; Sacre, J.W.; Straznicky, N.E.; Cohen, N.D.; Cerin, E.; Lambert, G.W.; Owen, N.; Kingwell, B.A.; et al. Benefits for Type 2 Diabetes of Interrupting Prolonged Sitting with Brief Bouts of Light Walking or Simple Resistance Activities. Diabetes Care 2016, 39, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Benatti, F.B.; Ried-Larsen, M. The Effects of Breaking up Prolonged Sitting Time: A Review of Experimental Studies. Med. Sci. Sports Exerc. 2015, 47, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Quan, M.; Xun, P.; Wu, H.; Wang, J.; Cheng, W.; Cao, M.; Zhou, T.; Huang, T.; Gao, Z.; Chen, P. Effects of interrupting prolonged sitting on postprandial glycemia and insulin responses: A network meta-analysis. J. Sport Health Sci. 2021, 10, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Saunders, T.J.; Atkinson, H.F.; Burr, J.; MacEwen, B.; Skeaff, C.M.; Peddie, M.C. The Acute Metabolic and Vascular Impact of Interrupting Prolonged Sitting: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 2347–2366. [Google Scholar] [CrossRef]
- Buffey, A.J.; Herring, M.P.; Langley, C.K.; Donnelly, A.E.; Carson, B.P. The Acute Effects of Interrupting Prolonged Sitting Time in Adults with Standing and Light-Intensity Walking on Biomarkers of Cardiometabolic Health in Adults: A Systematic Review and Meta-analysis. Sports Med. 2022, 52, 1765–1787. [Google Scholar] [CrossRef]
- Andersson, A.; Sjodin, A.; Olsson, R.; Vessby, B. Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am. J. Physiol. 1998, 274, E432–E438. [Google Scholar] [CrossRef]
- Prats, C.; Helge, J.W.; Nordby, P.; Qvortrup, K.; Ploug, T.; Dela, F.; Wojtaszewski, J.F.P. Dual Regulation of Muscle Glycogen Synthase during Exercise by Activation and Compartmentalization. J. Biol. Chem. 2009, 284, 15692–15700. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Welton, N.J. Network meta-analysis: A norm for comparative effectiveness? Lancet 2015, 386, 628–630. [Google Scholar] [CrossRef] [PubMed]
| Study | Participants | Age | Arms | Outcomes |
|---|---|---|---|---|
| Altenburg et al. [34] 2019, The Netherlands | 20 healthy-weight males | 19.2 ± 0.6 | Sitting 300 min | Insulin AUC |
| Interrupted with 10 min standing every 60 min | ||||
| Bailey et al. [36] 2015, UK | 10 non-obese healthy adults | 24 ± 3 | Sitting 300 min | Glucose AUC |
| Interrupted with 2 min bouts of standing every 20 min | ||||
| 2 min bouts of light-intensity walking (3.2 km/h) every 20 min | ||||
| Bailey et al. [35] 2016, UK | 13 healthy adults | 26.6 ± 8.5 | Sitting 300 min | Glucose iAUC; Insulin iAUC |
| 2 min bouts of light-intensity walking (3.2 km/h) every 20 min | ||||
| 2 min bouts of moderate walking (5.8–7.9 km/h) every 20 min | ||||
| Bailey et al. [20] 2022, UK | 12 overweight/obese adults | 48 ± 10 | Uninterrupted sitting ≥10 h | Glucose iAUC |
| Interrupted with 6–10 min of activity accrued in each hour | ||||
| Benatti et al. [43] 2017, Denmark | 14 inactive, healthy males | 30.1 ± 8.8 | Sitting 9 h | Glucose iAUC; Insulin iAUC; TAG iAUC |
| Interrupted by 15 min of standing every 30 min | ||||
| A single 30 min bout of moderate-intensity exercise on treadmill | ||||
| Bhammar et al. [13] 2017, USA | 10 overweight/obese adults | 32 ± 5 | Sitting 540 min | Glucose time-averaged CGMS |
| Sitting and a single 60 min bout of moderate walking | ||||
| Interrupted with 2 min moderate walking every 20 min | ||||
| Interrupted with 2 min vigorous walking every 60 min | ||||
| Blankenship et al. [21] 2014, USA | 10 overweight/obese | 51.9 ± 15.4 | Sitting 6 h, 30 min walking, ~300 kcal before lunch | Glucose AUC; Insulin AUC |
| Frequent breaks (every 20 min), ~300 kcal | ||||
| Champion et al. [44] 2018, UK | 24 inactive adults | 35.8 ± 10.9 | Sitting 390 min | Glucose iAUC; Insulin iAUC; TAG iAUC |
| Interrupted with 20 min of light-intensity walking every 60 min | ||||
| Charlett et al. [37] 2021, UK | 12 normal-weight | 25 ± 6 | Sitting 300 min | Glucose iAUC |
| 3 min of bodyweight resistance exercise every 30 min | ||||
| Chen et al. [32] 2018, UK | 11 centra overweight | 50 ± 5 | Sitting 315 min | Glucose iAUC; Insulin iAUC; TAG iAUC |
| 2 min walking (6.4 km/h) every 20 min over 315 min, 30 min total | ||||
| Chrismas et al. [22] 2019, Qatar | 11 obese females | 21–44 | Sitting 300 min | Glucose iAUC; Insulin iAUC; TAG iAUC |
| Interrupted with 3 min of moderate-intensity walking every 30 min | ||||
| Dunstan et al. [11] 2012, Australia | 19 overweight/obese | 53.8 ± 4.9 | Sitting 420 min | Glucose iAUC; Insulin iAUC |
| Interrupted with 2 min of light-intensity walking every 20 min | ||||
| 2 min bouts of moderate-intensity walking every 20 min | ||||
| Duvivier et al. [38] 2013, The Netherlands | 18 healthy adults | 21 ± 2 | Sitting 840 min | Glucose AUC; Insulin AUC |
| Sitting 780 min/day and 60 min of vigorous exercise | ||||
| Duvivier et al. [23] 2017, The Netherlands | 24 overweight/obese | 64 ± 7 | Sitting 13.5 h | Glucose iAUC; Insulin iAUC |
| Interrupted every 30 min with standing/walking bouts | ||||
| Hansen et al. [39] 2016, Denmark | 14 healthy normal weight | 20–23 | Sitting 150 min | Glucose iAUC |
| 2 min bouts of light-intensity walking (3.5–4.5 km/h) every 20 min | ||||
| Hawari et al. [24] 2019, UK | 14 overweight/obese | 37 ± 16 | Sitting 390 min | Insulin time-averaged AUC |
| Interrupted with 30 s of 10 chair squats every 20 min | ||||
| Kashiwabara et al. [53] 2018, Japan | 12 older women with hypertriglyceridemia, inactive | 70.5 ± 4.6 | Sitting 8 h | Glucose iAUC; Insulin iAUC; TAG iAUC |
| Moderate walking in one 30 min bout in the morning | ||||
| Light walking in twenty 90-s bouts (every 20 min) | ||||
| Kerr et al. [27] 2017, USA | 10 overweight/ obese postmenopausal women | 66 ± 9 | Sitting 300 min | Glucose iAUC; Insulin iAUC |
| 2 min of standing every 20 min | ||||
| 2 min of light-intensity walking every 60 min | ||||
| Larsen et al. [4] 2015, Australia | 19 overweight/obese | 56.7 ± 1.5 | Sitting 420 min | Glucose iAUC; Insulin iAUC; TAG iAUC |
| 2 min bouts of walking every 20 min (3.2 km/h) | ||||
| Ma et al. [54] 2020, China | 16 non-obese, inactive, healthy | 24 ± 3 | Sitting 540 min | Glucose iAUC |
| 3 min bouts of moderate walking (60%VO2max) every 30 min | ||||
| 5 min bouts of moderate walking (60%VO2max) every 45 min | ||||
| 8 min bouts of moderate walking (60%VO2max) every 60 min | ||||
| Maylor et al. [46] 2019, UK | 14 inactive females | 33.8 ± 13.4 | Sitting 450 min | Insulin iAUC |
| 2 min of moderate treadmill physical activity every 30 min | ||||
| McCarthy et al. [28] 2017, UK | 13 obese adults | 66 ± 6 | Sitting 7.5 h | Glucose iAUC; Insulin iAUC |
| 5 min arm ergometry every 30 min, total 1 h | ||||
| McCarthy et al. [40] 2017, UK | 34 healthy adults | 40 ± 9 | Sitting 7.5 h | Glucose iAUC; Insulin iAUC |
| 5 min light walking bouts every 30 min, total 1 h | ||||
| Miyashita et al. [52] 2016, Japan | 15 postmenopausal women | 68.8 ± 3.2 | Sitting 8 h | Glucose iAUC; Insulin iAUC |
| Sitting 1 h, 20 × 1.5 min walking every 15 min (3.7 km/h) | ||||
| Sitting 1 h, 30 min walking (3.7 km/h), 6.5 h sitting | ||||
| Newsom et al. [29] 2013, USA | 11 obese adults | 28 ± 2 | Sitting 480 min | Glucose AUC; Insulin AUC |
| Sitting and a single bout of exercise (~55 min, 65%VO2max) | ||||
| Peddie et al. [41] 2013, New Zealand | 70 normal-weight adults | 25.9 ± 5.3 | Sitting 9 h | Glucose iAUC; Insulin iAUC; TAG iAUC |
| Sitting 8.5 h and 100-s bouts of brisk walking (60% of VO2max) every 30 min | ||||
| Sitting 0.25 h, 30 min treadmill walking @60% VO2max, 8.25 h sitting | ||||
| Peddie et al. [42] 2021, New Zealand | 18 healthy, normal weight | 23.5 ± 5 | Sitting 6 h | Glucose iAUC; Insulin iAUC |
| 2 min walking (5 km/h, 10% incline) every 30 min | ||||
| Pulsford et al. [47] 2017, UK | 25 inactive males | 40.2 ± 12.2 | Sitting 420 min | Glucose AUC; Insulin AUC |
| 2 min bouts of light-intensity walking (3.2 km/h) every 20 min | ||||
| 2 min bouts of standing every 20 min | ||||
| Wheeler et al. [30] 2020, Australia | 67 overweight/obese | 67 ± 7 | Sitting 8 h | Glucose AUC; Insulin AUC |
| sitting 1 h, moderate-intensity walking (30 min), uninterrupted sitting 6.5 h | ||||
| Wong et al. [33] 2021, China | 21 young centrally obese males | 23 ± 4 | Sitting 360 min | Glucose iAUC; Insulin iAUC; TAG iAUC |
| 2 min bouts of light-intensity walking (3.2 km/h) every 30 min | ||||
| 6 min bouts of light-intensity walking (3.2 km/h) every 60 min | ||||
| Yates et al. [31] 2020, UK | 60 overweight/obese | 67–75 | Sitting 7.5 h | Time-averaged AUC for Glucose, Insulin, TAG |
| 5 min of self-paced light walking every 30 min | ||||
| Henson et al. [25] 2016, UK | 22 overweight/obese postmenopausal women | 66.6 ± 4.7 | Sitting 7.5 h | Insulin iAUC; TAG iAUC |
| 5 min bouts of standing every 30 min | ||||
| 5 min bouts of light-intensity walking every 30 min | ||||
| Di Pietro et al. [45] 2013, USA | 10 Inactive older impaired fasting glucose | 69 ± 6 | Sitting | Glucose time-averaged CGMS |
| One bout of 45 min morning walking (moderate intensity) | ||||
| Three 15 min bouts of moderate postmeal walking | ||||
| Duvivier et al. [49] 2017, The Netherlands | 19 T2DM | 63 ± 9 | Sitting 14 h | Glucose time-averaged CGMS |
| Sitting 13 h + 1 h moderate cycling (5.9 METs) | ||||
| Interrupted with light-intensity walking and standing every 30 min | ||||
| Dempsey et al. [55] 2016, Australia | 24 T2DM | 62 ± 6 | Sitting 7 h | Glucose time-averaged CGMS; Insulin iAUC; TAG iAUC |
| 3 min bouts of light-intensity walking at 3.2 km/h every 30 min | ||||
| 3 min bouts of simple resistance activities every 30 min | ||||
| Honda et al. [50] 2016, Japan | 16 T2DM | 65.4 ± 1.1 | Sitting | Glucose AUC |
| 3 min bouts of stair climbing up and down (80–110 steps/min) at 60 and 120 min | ||||
| van Dijk et al. [51] 2013, The Netherlands | 20 T2DM males | 64 ± 1 | Sitting 11 h | Glucose time-averaged CGMS |
| A single 45 min cycling at 50% max workload capacity (6 METs) | ||||
| Sitting and 3 × 15 bouts of walking after each 3 meals (3 METs) | ||||
| Holmstrup et al. [26] 2014, USA | 11 young, obese, impaired glucose tolerance | 25 ± 2.6 | Sitting 720 min | Glucose iAUC |
| A single 60 min bout of moderate-intensity exercise | ||||
| 5 min bouts of moderate-intensity exercise every 60 min |
| Outcome | Interruption (vs. Prolonged Sitting) | N of Studies | N of People | Effect Estimates | I2 (%) |
|---|---|---|---|---|---|
| Type/intensity | |||||
| SMD (95%CI) | |||||
| Blood glucose | MVPA | 7 | 147 | −0.6 (−0.83, −0.37) | 30.91 |
| LPA | 18 | 339 | −1.45 (−2.32, −0.57) | 96.4 | |
| Standing | 8 | 133 | −0.65 (−1.21, −0.09) | 81.24 | |
| RE | 3 | 49 | −1.04 (−1.58, −0.49) | 97.65 | |
| Insulin | MVPA | 9 | 175 | −0.53 (−0.73, −0.32) | 0 |
| LPA | 15 | 244 | −1.04 (−1.53, −0.55) | 85.99 | |
| Standing | 8 | 151 | −0.45 (−0.97, 0.06) | 80.37 | |
| RE | 2 | 37 | −0.86 (−1.34, −0.39) | 74.51 | |
| Triacylglycerol | MVPA | 3 | 90 | 0.22 (−0.07, 0.52) | 75.46 |
| LPA | 8 | 182 | −0.39 (−0.85, 0.06) | 81.46 | |
| Standing | 5 | 118 | 0.42 (0.17, 0.68) | 0 | |
| RE | 1 | 24 | −0.67 (−1.24, −0.1) | / | |
| Duration of breaks | |||||
| SMD (95%CI) | |||||
| Blood glucose | More than 5 min | 3 | 57 | −1.12 (−1.51, −0.73) | 32.82 |
| 5 min | 4 | 129 | −0.99 (−1.53, −0.45) | 83.49 | |
| 3 min | 5 | 87 | −0.88 (−1.31, −0.46) | 96.69 | |
| 2 min | 15 | 170 | −0.85 (−1.31, −0.39) | 78.82 | |
| Insulin | More than 5 min | 4 | 79 | 0.04 (−0.27, 0.34) | 0 |
| 5 min | 4 | 129 | −1.07 (−1.66, −0.49) | 85.87 | |
| 3 min | 3 | 35 | −0.91 (−1.35, −0.48) | 58.32 | |
| 2 min | 14 | 215 | −0.87 (−1.25, −0.50) | 72.66 | |
| Triacylglycerol | More than 5 min | 3 | 59 | −0.24 (−0.6, 0.12) | 50.38 |
| 5 min | 2 | 82 | −0.02 (−0.3, 0.26) | 91.81 | |
| 3 min | 2 | 35 | −0.39 (−0.81, 0.02) | 0 | |
| 2 min | 5 | 131 | −0.1 (−0.35, 0.14) | 82.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Pan, Y.; Zhang, X.; He, Q.; Chen, S.; Du, L.; Yin, S. Impact of Prolonged Sitting Interruption on Blood Glucose, Insulin and Triacylglycerol in Adults: A Systematic Review and Meta-Analysis. Appl. Sci. 2024, 14, 3201. https://doi.org/10.3390/app14083201
Dong Y, Pan Y, Zhang X, He Q, Chen S, Du L, Yin S. Impact of Prolonged Sitting Interruption on Blood Glucose, Insulin and Triacylglycerol in Adults: A Systematic Review and Meta-Analysis. Applied Sciences. 2024; 14(8):3201. https://doi.org/10.3390/app14083201
Chicago/Turabian StyleDong, Yelei, Yang Pan, Xianliang Zhang, Qiang He, Si Chen, Litao Du, and Shuting Yin. 2024. "Impact of Prolonged Sitting Interruption on Blood Glucose, Insulin and Triacylglycerol in Adults: A Systematic Review and Meta-Analysis" Applied Sciences 14, no. 8: 3201. https://doi.org/10.3390/app14083201
APA StyleDong, Y., Pan, Y., Zhang, X., He, Q., Chen, S., Du, L., & Yin, S. (2024). Impact of Prolonged Sitting Interruption on Blood Glucose, Insulin and Triacylglycerol in Adults: A Systematic Review and Meta-Analysis. Applied Sciences, 14(8), 3201. https://doi.org/10.3390/app14083201








