Abstract
Background: Baobab fruit is valued for its nutritional and medicinal benefits. Although it is acknowledged that baobab pulp is beneficial for health, studies that link its nutraceutical properties to the ability to eliminate reactive species (ROS and RNS) are scarce. Methods: The nutritional profile and the antioxidant properties of baobab pulp extracts from Angola were evaluated. Thus, for the first time, the evaluation of in vitro scavenging capacity against the most physiologically relevant reactive oxygen species (ROS) and reactive nitrogen species (RNS) were the focus of investigation. Results: Angolan fruit pulp presented high contents of ash (8.0%) and total dietary fiber (52%). Vitamin E content was reported for the first time in fruit pulp. Green solvents were used to quantify bioactive compounds and antioxidant activity. Hydroalcoholic extracts exhibited the highest contents of phenolics (1573.0 mg/100 g) and flavonoids (768.7 mg/100 g). Thus, hydroalcoholic extracts showed higher antioxidant activity, and higher scavenging capacity for ROS (O2•−, H2O2, HOCl, ROO•) and RNS (•NO, ONOO−), being most active for •NO and ONOO−. Conclusion: For the first time, Angolan baobab fruit was described in respect to its nutritional contribution as well as its positive antioxidant effects, both as a functional food and as a nutraceutical ingredient.
1. Introduction
Baobab (Adansonia digitata L.) is native to Sub-Saharan Africa. It is widely recognized for its socioeconomic significance for the local populations due to the plant’s many useful organs, which can be used for food, or for traditional medicine [1]. The Adansonia genus, a member of the Malvaceae family, encompasses eight identified species spread across numerous African countries [2]. Besides their nutritional importance, the protection of these wild fruits not only contributes to health but also yields economic advantages, as certain wild edible fruits are actively traded in various markets. Native plants, including fruits, are now used in several food recipes, feeds, medicinal applications, household products, and ritual applications, by the local population [3]. Thus, they play an important role in global food security, particularly in agricultural societies. Focusing on human health applications, several ethnobotanical studies have recorded the worldwide use of A. digitata for medicinal purposes [4,5]. Due to the existence of numerous compounds with biological action [6,7], this species, especially the fruit, has been reported to possess both nutritional and therapeutic value and it is therefore considered a food–medicine. The pulp of baobab fruit is acknowledged to be a valued source of minerals (calcium, potassium, iron, and magnesium), vitamins (ascorbic acid, niacin, and pyridoxine), and phenolic compounds like rutin, quercetin, catechin, gallic acid, and proanthocyanins [8,9,10]. According to Prior et al. [10], more polyphenols and vitamin C are found in baobab pulp than in similar commonly eaten fruits (kiwis, strawberries, oranges, and apples). However, taking into consideration the biological roles indicated in various research efforts, the fruit pulp lacks categorization regarding its other vitamin contents.
Also, fruit pulp possesses a high content of fibers and is low in proteins and fats [10]. Thus, baobab pulp appears to be ideal for adding fiber to foods, while also improving their nutritional profile [11,12,13]. Because of its higher content of soluble fiber, as compared to other foods, its nutritional profile indicates that baobab may be able to be categorized as a low-glycemic-index food [4,14]. In 2008, the European Commission added the pulp of baobab fruit to the list of novel food components in the EU, and, 1 year later, the Food and Drug Administration recognized it as a food ingredient [15]. Consequently, the nutritional benefit of baobab fruit has been recognized, and it has been accepted as a food additive in Europe and the United States of America [16]. Since then, an extensive amount of research has been conducted on its biochemical, agronomical, and botanical attributes. A significant variance in nutritional value has been reported, probably due to several factors, such as the origin, quality, production, storage, and so on of the analyzed samples, suggesting a need for more research to promote scientific knowledge on this species.
Baobab edible fruits are high in phytochemical compounds from an assortment of chemical families, including flavonoids, triterpenoids, steroids, and phenolic acids. Because of their antioxidant properties, bioactive compounds change and reduce the risk of chronic pathologies associated with oxidative stress. These compounds are associated with some of the reported biological activities, such as anti-hyperglycemic [15,17,18], analgesic and antipyretic [15,19], antibacterial, antiviral [15,20], antioxidant [18], and anti-inflammatory [4,10] properties. Moreover, polyphenols are well-known for reducing the worsening and/or development of oxidative stress. Reactive pro-oxidant species, particularly reactive oxygen species (ROS) and reactive nitrogen species (RNS), are overproduced during oxidative stress, leading to an inequity between pro-oxidants and antioxidants. To mitigate oxidative stress-related diseases, it becomes essential to introduce antioxidant molecules, aiming to restore and maintain optimal levels of antioxidants within the organism [21]. The potential scavenging capacity of methanolic extract of baobab fruit pulp against nitric oxide (NO) has already been reported in the literature [22]. However, despite the phytopharmaceutical interest in the antioxidant effects of baobab fruit pulp, no studies have been reported on ethanolic, aqueous, and hydroalcoholic extracts as to the evaluation of the in vitro scavenging effect against physiologically important ROS and RNS.
In recent years, there has been an increase of interest in baobab fruit, leading researchers to conduct studies on both edible and inedible parts of Adansonia digitata L. However, there are differences in the asserted values of baobab parts (pulp, seeds, and leaves), which could be attributed to a variety of factors, including different market samples, plant origin, soil and climacteric properties, and analytical methods, as well as the nature of the solvent extraction and the storage conditions, among other factors.
Therefore, this experimental work meant to analyze and report the proximate composition of baobab fruit pulp, as collected in several municipalities in the province of Luanda, Angola. Much of the recent research on baobab fruit has concentrated on regions in southern and eastern Africa, including Kenya and Sudan [8,19,22,23,24], South Africa [25], Mali [18], and Nigeria [8,17]. Although the economic interest in baobab fruit continues to increase, there is still a lack of knowledge on Angolan fruits. Vitamin E will be measured in the fruit pulp for the first time, with the goal of improving the antioxidant profile of baobab fruit and expanding its applications. Additionally, this research aims to assess the antioxidant activity in various baobab pulp extracts based on green solvents, and characterized by low toxicity, convenient accessibility, and great efficiency. Although prior research has been conducted on the antioxidant capacity of this fruit [5,9,15,17,19,22], we believe that this is a pioneering study on the evaluation of the in vitro scavenging effect of baobab extracts against physiologically important ROS and RNS. Furthermore, this study is noteworthy, considering that ROS and RNS are central in the cellular and biochemical process complexity of oxidative stress, which can be physiological (acting pro-hermetic) or pathogenic (producing destructive vicious circles) processes.
2. Materials and Methods
2.1. Raw Material and Reagents
Adansonia digitata L. fruits were collected from traditional local markets in three main municipalities of Luanda, Angola (Quiçama: 9.7984° S, 13.7289° E; Icolo Bengo: 9.1871° S, 13.8216° E; and Belas: 9.0834° S, 13.1279° E). Luanda has a dry tropical climate, and its typical soil consists of luvisols, calcisols, and cambisols (calcareous and calcialitic soils), which give good clayey soils for crops. Before analysis, the fruits were blended to create a representative sample from the Luanda region. A 2 mm sieve was used to separate the fruit pulp from the seeds. Following that, the fruit pulp was kept at room temperature (25–28 °C) in a hermetically sealed vial covered with aluminum foil until analysis.
All chemicals and solvents used were of analytical grade. 2,2-Diphenyl-1-picryl-hydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), ferrous sulphate heptahydrate, sodium carbonate decahydrate, sodium nitrite, aluminium chloride, and ferric chloride hexahydrate were all purchased from Aldrich Chemical Co. (Steinheim, Germany). Catechin and gallic acid were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Dihydrorhodamine 123 (DHR), sodium hypochlorite solution (NaClO) with 4% available chlorine, 3-(aminopropyl)-1-hydroxy-3-isopropyl-2-oxo-1-triazene (NOC-5), β-nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium chloride (NBT), phenazine methosulphate (PMS), lucigenin, α,α’-azodiisobutyramidine dihydrochloride (AAPH), fluorescein sodium salt, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), Tiron, ascorbic acid, and quercetin were all obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). 4,5-Diaminofluorescein (DAF-2), 30% hydrogen peroxide (H2O2), Folin–Ciocalteu’s reagent, absolute ethanol, sodium acetate, and sodium hydroxide were obtained from Merck (Darmstadt, Germany). Ultrapure water was obtained using a Milli-Q water purification system (TGI Pure Water Systems, Leominster, MA, USA). The peroxynitrite anion (ONOO−) was obtained by synthesis, as described by Gomes et al. [26]
2.2. Nutritional Profile
The nutritional analyses were performed using AOAC protocols [27]. Moisture content was measured using an infrared balance (Scaltec model SMO01, Scaltec Instruments, Heiligenstadt, Germany). The ashes were measured by direct incineration at 500 °C. Total fat and crude proteins were measured by the Soxhlet extraction and Kjeldahl methods, respectively. Dietary fiber analysis was carried out using enzymatic–gravimetric techniques [27]. The energy evaluation of fruit pulp (kcal/100 g) was calculated using conversion factors based on general Atwater values for food energy content in EU Regulation N. 1169, 2011 [28], being 4.0 kcal/g for protein and carbohydrates, and 9.0 kcal/g for fat.
2.3. Lipidic Fraction Extraction
The lipidic fraction of baobab fruit pulp was extracted according to the procedure validated by Alves et al. [29], with a few modifications. The sample (about 150 mg) was combined with 75 μL of 0.1% BHT (m/v), 50 μL of tocol (internal standard, 0.1 mg/mL), and 1 mL absolute ethanol. The solution was homogenized for 30 min using an orbital vortex mixer (VV3, VWR International, Darmstadt, Germany). After that, 2 mL of n-hexane (HPLC grade) was added, and the solution was homogenized for 30 min. Next, 1 mL of 1% NaCl (m/v) was added. The supernatant was collected and preserved following centrifugation (5000 rpm for 5 min) with a Heraeus Labofuge A (Hanau, Germany). The residue was re-extracted with 2 mL of n-hexane for 30 min before centrifugation (5000 rpm for 5 min). The resultant supernatant was collected and mixed with the previous extract. An appropriate amount of anhydrous sodium sulphate (Na2SO4) was added to the solution, which was then centrifuged (5000 rpm for 5 min). After being evaporated to dryness, the supernatant was mixed with 1 mL of solvent and injected into an HPLC-DAD-FLD (high-performance liquid chromatography connected to a diode array detector and fluorescence detector) system for assessment of the vitamin E profile.
Vitamin E Profile by HPLC-DAD-FLD
Vitamin E quantification was carried out through HPLC-DAD-FLD, employing an integrated HPLC system (Jasco, Tokyo, Japan) that included an AS-950 automated injector, a PU-980 pump, and an MD-2015 multiwavelength diode array detector (DAD) coupled to an FP-2020 fluorescence detector (Jasco, Tokyo, Japan). The system was programmed for excitation at 290 nm and emission at 330 nm. Each compound was chromatographically separated on a normal phase SupelcosilTM LC-SI column (75 mm × 3.0 mm, 3.0 μm) (Supelco, Bellefonte, PA, USA), following the method described by Alves et al. [29]. Chromatographic data analysis was conducted using Borwin-PDA Controller Software (Jasco ChromNAV, version 2.02.08, Tokyo, Japan).
Vitamin E vitamers in samples were identified by comparing the retention times with the respective standards (α-, β-, γ-, and δ-tocopherols and α-, β-, γ-, and δ-tocotrienols), using UV spectra as a reference. Quantification was made through the analysis of fluorescent signals, and subsequently converted to concentration units using calibration curves obtained from commercial standards for each compound. Tocol was used as the internal standard. The results were expressed as mg/100 g of sample dry weight (dw).
2.4. Total Phenolic and Total Flavonoids Content
2.4.1. Preparation of Baobab Pulp Extracts
Samples (~1 g) were macerated with 20 mL of ethanol, ethanol/water (1:1), or distilled water for 30 min at 40 °C, based on the parameters established out by Costa et al. [30]. The extracts were stored at 4 °C until evaluated. Aqueous filtrates were freeze-dried, and hydroalcoholic and alcoholic filtrates were reduced to residue by a rotary evaporator at 40 °C. The extracts were reconstituted in the extraction solvent to determine the bioactive compounds and antioxidant activity.
2.4.2. Total Phenolic Contents and Total Flavonoid Contents
The total soluble phenolic content (TPC) of the extracts was determined by employing the Folin–Ciocalteu (FC) solution, with some adjustments made according to the approach described by Vinha et al. [31]. Succinctly, 30 μL of each extract was mixed with 150 μL of FC reagent (1:10) and 120 μL of Na2CO3 aqueous solution (7.5%, m/v). The mixture was initially kept at 45 °C for 15 min; this was followed by 30 min of incubation without the presence of light, and at room temperature. A Synergy HT Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA) was used to obtain absorbance measurements (765 nm). The amount of TPC was calculated using a gallic acid calibration curve (covering the concentration range between 5 and 300 µg/mL), and results were presented in milligrams of gallic acid equivalents (GAE) per 100 g of dry weight (dw).
A colorimetric test was used to determine the total flavonoid contents (TFC), using the conditions previously published by Costa et al. [32]. To summarize, 1 mL of each sample extract was combined with 4 mL of distilled water and 300 μL of 5% NaNO2. A total of 300 μL of 10% AlCl3 was added after 5 min at room temperature. One minute later, 2 mL of NaOH) (1 M) and 2.4 mL of distilled water were added. Catechin was used to plot a standard curve (2.5–400 mg/L; R2 = 0.999), and absorbance was measured at 510 nm using a Synergy HT Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA). The results were presented in milligrams of catechin equivalent (CAE)/100 g dw.
2.5. Antioxidant Activity
2.5.1. Determination of DPPH Free Radical Scavenging
The DPPH scavenging ability of the different extracts was determined according to the method validated by Costa et al., with modifications [30]. Briefly, 30 μL of Trolox standard (562 µg/L)/blank solution or diluted extract (1:10) were mixed with 270 μL of the DPPH• solution (6.1 × 10−5 M). The kinetic reaction was monitored with a Synergy HT Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA), in equal intervals of 10 min, at 525 nm. The reaction endpoint was attained in 20 min. Trolox was used as a standard in the preparation of the calibration curve (2.5–1000 µg/mL, R2 > 0.996) and DPPH• scavenging activity was expressed in mg of Trolox equivalents (TE)/100 g of sample (dw).
2.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay was carried out according to Costa et al. [30], with a few adjustments. In short, 35 μL of ferrous sulphate standard (5–600 μmol)/blank/diluted extract (1:10) was combined with 265 μL of the FRAP reagent (containing 0.3 M acetate buffer, 10 mM TPTZ solution, and 20 mM of FeCl3). The mixture was kept away from light for 30 min at 37 °C, and then the absorbance was measured at 595 nm using a Synergy HT Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA). The standard curve was constructed using FeSO4 (150–2000 µmol, R2 > 0.996), and the results were expressed as μmol FeSO4 (FSE) equivalents/100 g (dw).
2.6. ROS and RNS Scavenging Assays
The ROS [(O2•−), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), and peroxyl radical (ROO•)] and RNS [nitric oxide (•NO), and ONOO•] scavenging assays were performed using a microplate reader (Synergy HT, BioTek Instruments, Inc., Winooski, VT, USA) equipped with a thermostat, for fluorescence, UV/Vis, and chemiluminescence measurements. In these experiments, baobab fruit pulp extracts and the positive controls quercetin, Tiron, Trolox, and ascorbic acid were dissolved in the buffer used for each assay. The results were expressed as IC50 values of four independent experiments using five to seven concentrations in duplicate. The IC50 values were determined using curves of percentage inhibition versus compound concentration.
2.6.1. O2•−-Scavenging Assay
A non-enzymatic system (NADH/PMS/O2) created O2•−. These radicals form a purple-colored medium via the reduction of the NBT into diformazan [26], enabling the spectroscopic measurement of O2•− scavenging capacity at 560 nm. Tiron and ascorbic acid served as positive controls. The results were expressed as the inhibition (in IC50, except for ascorbic acid, whose result was presented as a percentage) of the NBT reduction to diformazan.
2.6.2. H2O2-Scavenging Assay
The scavenging activity of the H2O2 was determined according to a methodology previously described [33]. The effects of the studied extracts were monitored to establish the latter’s H2O2-scavenging activity (31.25–1000 µg/mL) and the effect of the positive control (ascorbic acid) (31.25–1000 µg/mL) on the H2O2-induced oxidation of lucigenin. The results were expressed as the inhibition (in percentage for baobab pulp extracts and in IC50 for positive controls) of the H2O2-induced oxidation of lucigenin.
2.6.3. HOCl-Scavenging Assay
The HOCl scavenging capacity was measured by a fluorescent methodology described by Gomes et al. [26], one based on monitoring the effects of the extracts (aqueous extract: 31.25–1000 µg/mL; hydroalcoholic extract: 7.81–250 µg/mL; and alcoholic extract: 15.63–500 µg/mL) and the positive controls (ascorbic acid: 0.5–10 µg/mL; and quercetin: 0.08–5 µg/mL) on the HOCl-induced oxidation of DHRc to rhodamine 123. HOCl was generated by adjusting the pH of 1% NaClO solution (m/v) with H2SO4 (10%) to 6.2. The HOCl concentration was obtained in a spectrophotometer at 235 nm. The results were established as the inhibition (in IC50) of HOCl-induced oxidation of DHR.
2.6.4. ROO•-Scavenging Assay
ROO• scavenging capability was assessed by monitoring the effects of the examined extracts (aqueous and hydroalcoholic extracts: 31.25–1000 µg/mL; and alcoholic extract: 15.63–500 µg/mL) and the positive controls (ascorbic acid: 0.05–1.5 µg/mL; and quercetin: 0.006–0.2 µg/mL) on the fluorescence decay resulting from ROO•-induced oxidation of fluorescein [34]. ROO• was produced by the thermal degradation of AAPH at 37 °C. The fluorescence signal was monitored every minute at the emission wavelength of 528 ± 20 nm and excitation was performed at 485 ± 20 nm until the complete discoloration of fluorescence. Trolox was employed as a standard control, and the results were expressed as ROO•-induced oxidation of fluorescein.
2.6.5. •NO-Scavenging Assay
The ability to scavenge the •NO was tested by monitoring the effects of baobab pulp extracts (7.81–500 µg/mL) and the positive control (quercetin: 0.06–2 µg/mL) on •NO-induced oxidation of DAF-2 (non-fluorescent) to triazolofluorescein (DAF-2T) (fluorescent) [35]. The •NO was produced via the degradation of NOC-5. The results are presented as the percentage inhibition of •NO-induced oxidation of DAF-2.
2.6.6. ONOO−-Scavenging Assay
ONOO−-Scavenging capacity was determined by monitoring the effects of the baobab pulp extracts (aqueous extract: 7.81–500 µg/mL; hydroalcoholic extract: 1.95–125 µg/mL; alcoholic extract: 7.81–250 µg/mL) and the positive controls (ascorbic acid: 0.16–2.5 µg/mL; quercetin: 0.31–10 µg/mL) on ONOO−-induced oxidation of DHR (non-fluorescent) to rhodamine 123 (fluorescent) [33]. The experiments were performed in the presence and absence of 25 mM NaHCO3. The results are displayed as the % inhibition of ONOO−-induced oxidation of DHR.
2.7. Statistical Analysis
The collected data were statistically analyzed using IBM SPSS Statistics (version 26 for Windows, IBM Corp., Armonk, 241 NY, USA). One-way ANOVA was used to assess significant differences between samples, followed by Tukey’s HSD, to make paired comparisons between means, with a significance level of 5% (p ≤ 0.05). The correlation values between bioactive substances (TPC and TFC) and antioxidant activity (DPPH and FRAP) were examined using Pearson correlation. Differences of p ≤ 0.05 were considered significant.
3. Results
3.1. Nutritional Profile
The nutritional composition of baobab fruit pulp was determined, as shown in Table 1.

Table 1.
Nutritional composition of baobab fruit pulp. Results are expressed in g/100 g dry weight (dw).
The baobab fruit pulp is a rather low-quality source of protein (2.3 ± 0.1%) and lipids, (0.4 ± 0.1%), and its most prevalent macronutrient is total dietary fiber (52.0 ± 1.0%). The ash content is high, indicating a high mineral concentration.
3.2. Vitamin E Profile
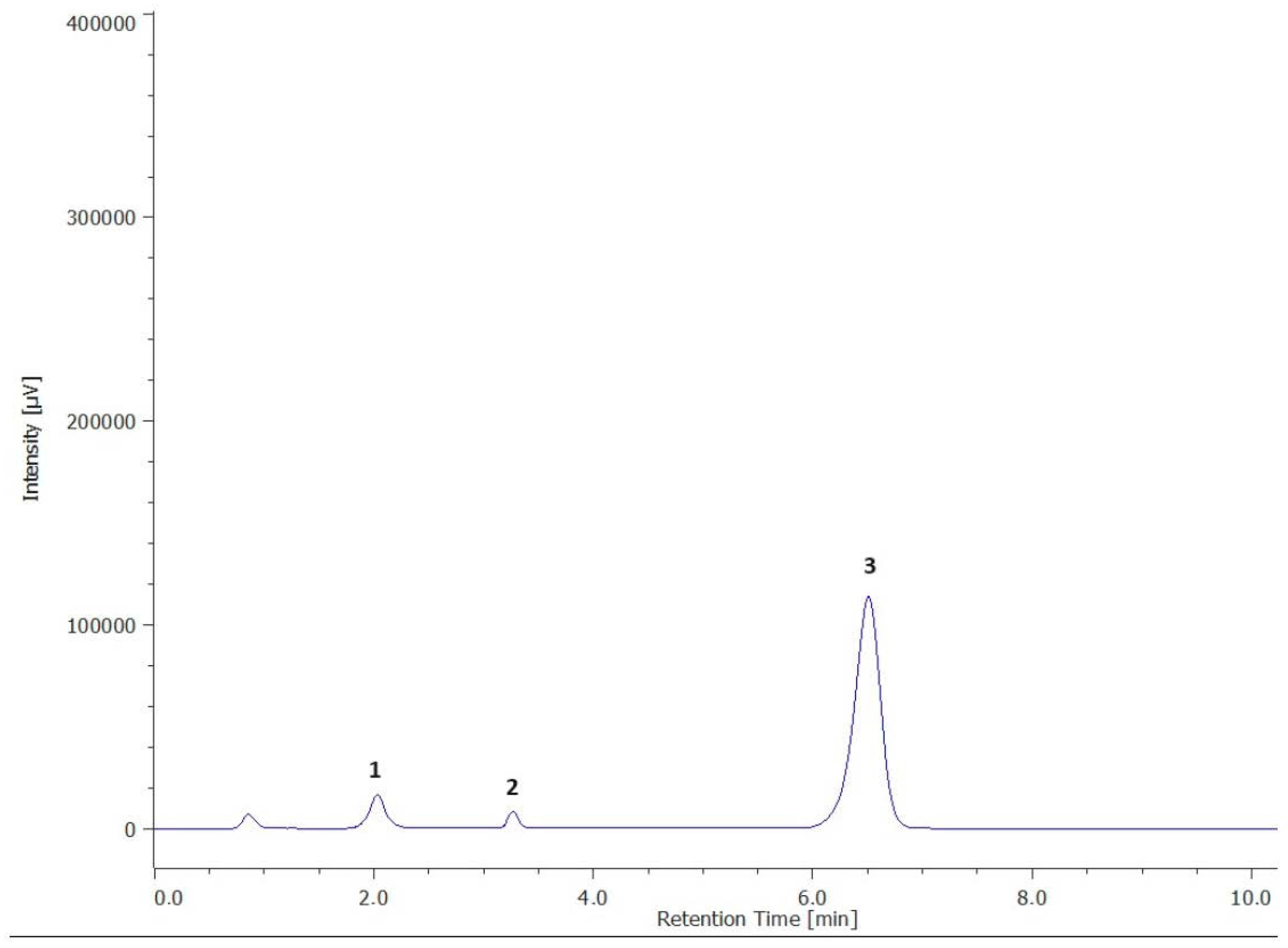
Vitamin E is utilized not just as a preservative in animal feed, but also as a medical ingredient, dietary supplement, and antioxidant food stabilizer. Considering the biological properties of vitamin E, as well as its antioxidant activity, this vitamin was quantified, for the first time, in this study in the pulp of the baobab fruit (Table 2, Figure 1).

Table 2.
Vitamin E profile of baobab fruit pulp. Results are expressed in mg/100 g dw.
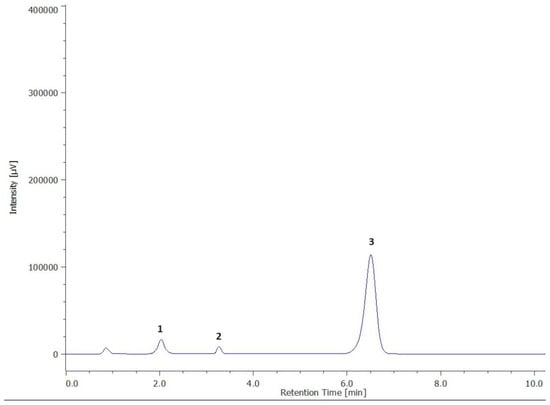
Figure 1.
Chromatogram of vitamin E quantification. (1—α-tocopherol; 2—β-tocopherol; 3—tocol-internal standard).
3.3. Bioactive Compounds and Antioxidant Activity
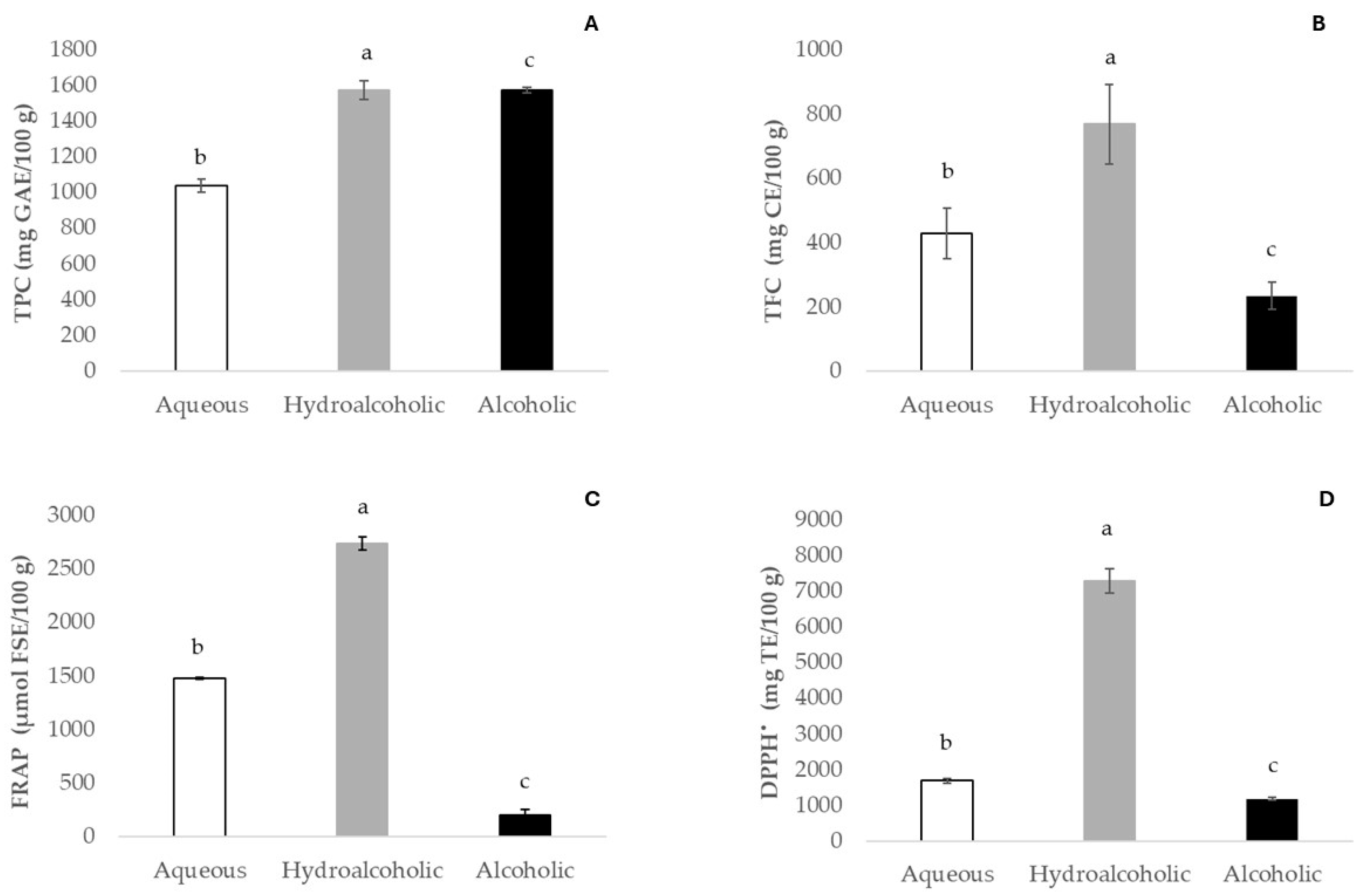
The evaluations of total phenolic and flavonoid contents and the antioxidant activity detected in fruit pulp extracts are presented in Figure 2.
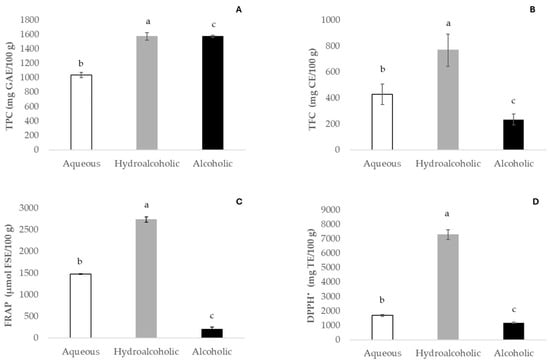
Figure 2.
Total phenolic content (TPC, (A)), total flavonoid content (TFC, (B)), ferric reducing antioxidant power (FRAP, (C)), and activity inhibition of 2,2-diphenyl-1-picrylhydrazyl (DPPH•, (D)). Values represent mean ± SD (n = 6). Results are expressed as mg of gallic acid equivalents (GAE), mg of catechin equivalents (CE), μmol of ferrous sulfate equivalents (FSE), and mg of Trolox equivalents (TE) per 100 g of baobab pulp. Different letters denote significant differences (p ≤ 0.05).
3.4. ROS and RNS Scavenging Assays
Table 3 shows the scavenging activity of baobab pulp extracts against the ROS (O2•−, H2O2, HOCl, and ROO•) and RNS (•NO and ONOO−).

Table 3.
Superoxide radical (O2•−), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), peroxyl radical (ROO•), nitric oxide (•NO), and peroxynitrite (ONOO−) scavenging activities of aqueous, hydroalcoholic, and alcoholic extracts from A. digitata pulp fruits and positive controls.
4. Discussion
4.1. Nutritional Profile
In this work, the centesimal composition of baobab fruit pulp was analyzed. Despite the high nutritional characterization of this fruit pulp [1,7,14,23,36], it is important to perform this analysis due to the fact that the nutritional content varies, depending on an assortment of factors, including geographic origin, soil and temperature conditions, and fruit maturation index, among other factors [23]. Until now, no research has been conducted on this fruit, as harvested in Luanda (Angola). Our results revealed high contents of total dietary fiber (52%) and total ash (8%) in baobab fruit pulp. Low protein and total fat contents were observed. The high quantity of total ash suggests that the fruit pulp is rich in inorganic matter, which is in accord with its high mineral content. Thus, the European Union has defined a range of 5.5–6.6% ash content for baobab pulp [15]. Our findings are consistent with the levels described in the literature, although slightly higher. According to several studies, baobab pulp contains substantial amounts of iron, zinc [36], magnesium, calcium, and potassium [23]. Considering geographical proximity, Monteiro et al. [14] described lower ash contents (4.71–5.18%) in Angolan baobab fruits collected from the municipalities of Namibe (southern Angola). However, the same authors reported identical contents of dietary fiber, ranging from 51.38% to 56.62%. The inorganic composition variation may be due to the adjustments of plant root architecture and exudation, in response to nutritional needs and soil conditions, with several modifications acting as markers of the plant’s and the soil’s nutritional status.
According to Muthai et al. [36], the baobab fruit pulp contains higher concentrations of all critical nutrients, compared to regularly consumed fruits including bananas, mangoes, and berries. Our results also showed that baobabs from the Luanda region possess an important economic value and are a good and low-cost source of total dietary fiber (52.0%). Regarding dietary fiber content, Muthai et al. [36] reported fiber levels ranging from 5.4% to 11.15% in several baobab fruits collected from distinct areas (Tanzania, Zambia, Kenya, Malawi, and Mali). Also, lower dietary fiber contents (20.62–24.19%) were described in baobab fruit pulp from two districts of Mozambique [37]. Other studies, however, report values ranging from 45.1% to 52%, values similar to those obtained in this study [9,14,24]. One possible reason justifying this variation may be the different evaluations: crude fiber or cellulose, rather than dietary fibers, which are not the same. Also, several analytical methods are used, like enzymatic gravimetric assay and the AOAC method, which can lead to differing assessments of the results. Despite everything, dietary fiber intake promotes favorable body weight and overall metabolic health. According to Barber et al. [38], increased dietary fiber consumption has also been associated with lower incidence of cardiovascular diseases. Additionally, there is evidence suggesting further health benefits of a diet rich in fiber, such as a lower risk of cancer, and a better gastrointestinal health [36], increasing the interest in baobab fruit. Regarding carbohydrate content, our sample exhibited a value of 37.0%, which is higher than those obtained by Monteiro et al. [14] in Angolan fruits. Higher values were reported in fruit pulp from Mozambique, ranging between 70.74–77.23% [37]. Since carbohydrates are usually determined by difference (indirect method), these variations in the amounts of carbohydrates come from the fact that the fiber content increases the apparent amount of digestible carbohydrates. Despite all of these differences, the baobab fruit pulp provides a balanced nutritional profile, which enhances its incorporation into food supplements, in addition to its other industrial applications. The protein amount of fruit pulp obtained was 2.3%, and therefore inside the range of 2.03–3.24% reported by European Union [38] with reference to a novel food ingredient. Moreover, our results agree with others described in the literature [23]. It should be acknowledged that baobab fruit is recognized for its low protein and fat contents [6]. The total fat content obtained in this work was very low (0.4%), indicating a probable similarity with previous research that did not address the fat content in fruits harvested in Angola and Kenya [14,23].
4.2. Bioactive Compounds and Antioxidant Activity
As a food source, fresh fruits and their derivatives offer vital dietary micronutrients as well as bioactive substances like vitamins and polyphenols. Vitamin C is an essential nutrient and an antioxidant compound described as being found in abundance in baobab fruit powder [23,39,40]. According to research described in the OJEU [39], the amount of vitamin C found in baobab fruit pulp is three times greater than that found in oranges. Despite the importance of vitamin C, few or no studies mention vitamin E, an outstanding natural antioxidant. In addition to its antioxidant properties, vitamin E is involved in immunological function, cell signaling, gene expression regulation, and other metabolic processes [41]. Thus, protein kinase C, an enzyme involved in smooth muscle cells, and platelet and monocyte proliferation and differentiation, is inhibited by alpha-tocopherol. Additionally, vitamin E raises the production of two enzymes that limit the metabolism of arachidonic acid, increasing the endothelium’s release of prostacyclin, which dilates blood vessels and prevents platelet aggregation [42]. Given its beneficial properties, the content of vitamin E was determined in our study. To our knowledge, no study has reported the vitamin E content in baobab fruit pulp. Only two vitamers were detected, α-tocopherol, and β-tocopherol (0.54 mg/100 g and 0.0509 mg/100 g, respectively). Even though the fruit pulp contained less vitamin E than vitamin C (163.8–288.9 mg/100 g), as described in other baobab fruits from Angola [14], this determination is innovative. Indeed, in addition to the health benefits of vitamin E as to human metabolism, the antioxidant activity of vitamin E may contribute to food preservation, reducing the negative impact of chemical reactions on food systems by improving the safety, nutritional value, and shelf-life of food products. Thus, our results may suggest the use of this fruit pulp as a functional ingredient for food products [43].
Regarding the polyphenolic contents in our samples, substantial variability in the TPC of baobab fruit pulp was observed among the different extracts in our study. The hydroalcoholic extract exhibited the highest concentration (1573.0 mg GAE/100 g), followed by the aqueous one (1038.6 mg GAE/100 g). As for the measurement of bioactive compounds (TPC and TFC), the ethanolic extract was revealed to be the worst extraction solvent. Tembo et al. [44] reported total phenolic contents of Malawi baobab pulp methanolic extracts ranging from 1870 to 4057.5 mg GAE/100 g. Another study on baobab fruit extracts from different locations of Sudan described phenolic contents ranging from 15.50 to 99.66 mg GAE/g [45]. Fruit pulp extracts from Madagascar presented 1090 mg GAE/100 g of total phenolic content [46], and those obtained in Burkina Faso showed a variation in total phenolic content between 3520 and 4060 mg GAE/100 g [15]. However lower total phenolic contents were reported in methanolic baobab fruits collected in Angola, ranging from 460.5 mg GAE/100 g to 972.2 mg GAE/100 g [14]. As previously stated, these differences can be related to both environmental conditions [23] and the nature of the extracting solvent. For instance, Ismail et al. [5] used different solvents on baobab fruit pulp and reported higher TPC values in acetone extract, when compared to methanolic extract. However, for aqueous and ethanolic extracts, the values reported by the same authors were significantly lower than those obtained in this work. According to earlier research, the nature of the solvent applied affects both the effectiveness of the extraction and the amounts of phenolic compounds extracted [15,44]. Additionally, extraction times and temperatures and the maceration or pulverization of the samples possibly influence the TPC results. Currently, there is no suggested solvent for the best recovery of phytochemicals, due to their diverse chemical characteristics and polarities, which may affect their solubility in a particular solvent. Phenolic compounds are usually extracted with organic solvents, referred to as conventional solvents, such as methanol, acetone, benzene, chloroform, petroleum ether, and hexane [47]. However, they are frequently rejected because of their flammability, explosiveness, low biodegradability, and toxicity to the final consumer. Green technologies are being tested, with a focus on efficacy and efficiency, increasing productivity, enhancing process selectivity, and reducing energy use. Alternative solvents, such as ionic liquid (IL) solvents, deep eutectic solvents (DESs), and natural eutectic solvents (NADESs), are a different option for this problem [47].
Just as with TPC, certain factors are observed with reference to the total flavonoid content (TFC). The profile of TFC extractive capacity recovery followed the same pattern as phenolics, with higher contents in hydroalcoholic extract (768.7 mg CAE/100 g), followed by aqueous (428.4 mg CAE/100 g) and ethanolic (235 mg CAE/100 g) extracts, respectively.
Our study has demonstrated that, on average, the total flavonoid concentration is always lower than the total phenolic concentration. These results contradict those reported by Ismail et al. [5] and Wasihun et al. [48], which reported higher amounts of flavonoids in most of the baobab pulp extracts. However, our results are in accordance with other studies [15,45]. Lamien-Meda et al. [15] reported TFC in baobab pulp of 31.70 ± 3.35 mg QE/100 g and 42.73 ± 0.46 mg QE/100 g, using methanol and acetone solvents, respectively, while total phenolic contents were significantly higher in both solvents (3518.33 and 4057.50 mg GAE/100 g, respectively). Furthermore, fruit ripening is a multifaceted, genetically engineered process that results in notable alterations regarding color, texture, flavor, and chemical composition. Kamatou et al. [49] observed a reduction in phenolic and flavonoid concentrations during the development and maturation of baobab fruit. Despite the above, in general, flavonoid content remains understudied, and the few studies that have described their content levels used different solvents.
The antioxidant activity of baobab fruit pulp extracts was assessed using the free radical scavenging capacity (DPPH•) and the ferric reducing antioxidant power (FRAP) assays. The values observed in both antioxidant activity assays were directly related to the bioactive contents obtained in each extract evaluated. Regarding the DPPH• assay, scavenging capacity ranged from 1189.3 to 7288.2 mg Trolox/100 g for alcoholic and hydroalcoholic extracts, respectively. In the FRAP assay, the hydroalcoholic extract exhibited the highest antioxidant activity (2736.7 μmol FSE/100 g), followed by the aqueous and alcoholic extracts (1477.8 and 199.2 μmol FSE/100 g, respectively). Ismail et al. [4] reported lower DPPH• values for baobab pulp extracts from Malawi and South Africa, ranging from 721.78 mg Trolox/100 g (ethanol) to 2089.76 mg Trolox/100 g (hydroalcoholic). Our results showed significant differences (p ≤ 0.05) in both antioxidant assays in aqueous, hydroalcoholic, and alcoholic extracts. To summarize, the antioxidant activity of baobab fruit extracts is related to total polyphenolic concentrations, and this was additionally found in our investigation. Pearson’s correlation was calculated, as polyphenols are predominantly responsible for antioxidant activity. TFC, FRAP, and DPPH all had exceptionally positive correlation coefficients with TPC, of 0.893, 0.991, and 0.836, respectively. Furthermore, the correlations between FRAP and DPPH relative to TFC were 0.980 and 0.941, respectively. Our results suggest that flavonoid contents contribute more to the overall antioxidant activity.
4.3. Reactive Oxygen and Nitrogen Species
One of the novelties of this work reports the evaluation of baobab fruit pulp extracts against reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and RNS play critical roles in the immune system. Moreover, oxidative stress is induced by an imbalance between the systemic synthesis of reactive pro-oxidant species and the biological system’s ability to quickly neutralize or repair the ensuing damage. Antioxidant compounds obtained from food or dietary supplements can reduce the generation of reactive species at different stages of oxidative stress and through different pathways [21,50]. Thus, traditional fruits, including baobab, might be acknowledged as significant healthy antioxidant sources [2,9,14].
The extracts’ scavenging abilities against several ROS and RNS were assessed. O2•− is the first reactive pro-oxidant specie produced because of the activity of multiple enzymes in the complex cascade of reactive species production. Once produced, O2•− can be physiologically dismutated to H2O2, either spontaneously under acidic conditions or through the action of the enzyme superoxide dismutase [51]. In turn, H2O2 can form hydroxyl radicals (HO•) through contact with transition metal ions. HO• is the most reactive and oxidative ROS, and it can induce lipid peroxidation with polyunsaturated fatty acids, resulting in the production of ROO•. After a reaction with chloride ions and in the presence of myeloperoxidase, H2O2 is also used to produce additional microbicide ROS, namely, HOCl [50,51]. A comprehensive review of the results obtained in this investigation revealed that, among the extracts examined, the hydroalcoholic extract has a higher scavenging effect against the assessed ROS and RNS (Table 3). In the O2•—scavenging assay, Tiron was the most effective scavenger, whereas ascorbic acid was the least effective.
Quercetin showed no activity in this assay up to the highest quantity tested (1000 μg/mL). Furthermore, the ROO•—scavenging capacity of baobab pulp fruit extracts was determined using the ORAC test. Nonetheless, none of the extracts demonstrated appreciable scavenging action for this ROS at the highest tested concentration (1 mg/mL). According to several studies, the cellular oxidative stress can be modulated by flavonoids by the scavenging of O2•−, neutralizing the effects of this ROS; through hydrogenation; or by complexing with oxidant species [21,50,52]. In terms of RNS-scavenging capacity, all of the extracts proved to be effective scavengers of both •NO and ONOO−. The hydroalcoholic extract was, once again, the most active of the extracts tested, with IC50 values of 27 ± 5, 19 ± 1, and 17 ± 1 μg/mL for •NO and ONOO− evaluated in the absence and presence of NaHCO3, respectively. The inclusion of NaHCO3 in the assays is crucial because, under physiological conditions, ONOO− rapidly reacts with CO2 (in equilibrium with bicarbonate anion), resulting in the formation of the nitrosoperoxycarbonate anion (ONOOCO2−). The subsequent decomposition of ONOOCO2− gives rise to various species, including nitrogen dioxide (NO2) and carbonate radical (CO3•−). Freitas et al. [53] stated that these substances are the main causes of nitration and/or oxidation processes that occur in vivo. Since ascorbic acid was previously found in significant concentrations in baobab pulp fruit, this isolated compound was also evaluated in similar assays to compare its results to those obtained for the extracts. When compared to the baobab pulp extracts, ascorbic acid demonstrated significantly greater scavenging capacity for almost all of the studied ROS and RNS (excluding O2•−). However, the scavenging capacity of quercetin for all of the assessed ROS and RNS was much greater than the values reported for the extracts. Nonetheless, quercetin was the most effective scavenger of H2O2, HOCl, ROO•, and •NO, outperforming ascorbic acid. Some in vitro and in vivo studies have demonstrated the efficacy of quercetin as an antioxidant in decreasing and avoiding damage and oxidative stress caused by free radicals [54]. HOCl, a highly oxidative reactive molecule naturally produced in neutrophils by myeloperoxidase, also yielded intriguing results. Rita et al. [55] reported a high scavenging capacity for O2•− and •NO radicals (IC50—77.14 ± 3.43 and 39.33 ± 6.89 μg/mL, respectively) in aqueous extracts of baobab fruit pulp, although significantly lower than our results. Ibraheem et al. [22], using methanolic extracts of A. digitata pulp, also stated a higher value for •NO inhibitory activity (IC50—36.55 ± 1.79 μg/mL). According to Ntchapda et al. [56], aqueous extract of baobab fruit reduces •NO levels in the aorta, heart, and kidney of hypertensive rats. Thus, according to Ibrahim et al. [57], the polysaccharides of A. digitata have stronger O2•− and •NO scavenging activity, as well as stronger H2O2 scavenging activity, than butylated hydroxytoluene (BHT) and ascorbic acid. Therefore, the results support our hypothesis that baobab pulp extracts, mainly aqueous and hydroalcoholic, possess an antioxidant capacity through the inhibition of ROS and RNS, which can lead to oxidative stress prevention. This study provided a novel approach for assessing the Angolan native Adansonia digitata L. species by emphasizing its potential as superfruit or alternatively as a functional ingredient.
5. Conclusions
Baobab pulp can be consumed as food or used as a functional ingredient, owing to its high content levels of ashes, dietary fiber, and phenolic and flavonoid compounds. This phytochemical profile is responsible for the high antioxidant activity reported in this work. Adansonia digitata L. health benefits encourage large-scale regional and global commercialization of this fruit. However, the quantities of nutrients and non-nutrients can be impacted by the location of the gathered baobab samples, as well as the extraction solvents and analytical procedures. For the first time in baobab fruit research, results were presented showing that the use of non-toxic green solvents allowed the production of extracts capable of scavenging ROS and RNS. Summarizing, the obtained results demonstrated that A. digitata fruit pulp is rich in bioactive compounds that may be related to its biological activity, which is characterized by evident antioxidant properties, corroborating the nutritional value of this delicacy. Further research is suggested, including enhanced sampling of the baobab fruits collected from different geographical areas of Angola. Several factors, like geographical origin, ripening stages, and agrotechnical measures may influence the nutritional and chemical composition of the fruits. Similarly, the identifications of individual phenolic compounds and their individual quantifications are relevant. Also, the in vitro antiproliferative and antitumor activities of fruit pulp extracts should be tested against human cancer cell lines to evaluate the bioactivity of the chemical compounds described in baobab fruit.
Author Contributions
Conceptualization, A.F.V. and A.S.G.C.; methodology, A.S.G.C., F.B.P., L.E.S., M.F., E.F. and A.F.V.; validation, A.F.V., A.S.G.C., M.F. and E.F.; formal analysis, L.E.S., A.F.V. and A.S.G.C.; investigation, A.F.V., F.B.P., M.F. and E.F.; resources, E.F. and M.B.P.P.O.; data curation, F.B.P., A.F.V. and A.S.G.C.; writing—original draft preparation, A.F.V., C.S. and M.B.P.P.O.; writing—review and editing, A.F.V., C.S., E.F. and M.B.P.P.O.; supervision, A.F.V., M.F., E.F. and M.B.P.P.O.; project administration, A.F.V. and M.B.P.P.O.; funding acquisition, E.F. and M.B.P.P.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work received support from FCT/MCTES (IUDB/50006/2020 DOI 10.54499/UIDB/50006/2020) through national funds.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Authors want to acknowledge support and help from FCT/MCTES (LA/P/0008/2020 https://doi.org/10.54499/LA/P/0008/2020, UIDP/50006/2020 https://doi.org/10.54499/UIDP/50006/2020 and UIDB/50006/2020 https://doi.org/10.54499/UIDB/50006/2020) through national funds. L. Espírito Santo expresses thanks for the REQUIMTE 2023-49 grant from LAQV (UIDB/50006/2020). M. Freitas acknowledges CEEC Individual 2020.04126.CEECIND/CP1596/CT0006 and LAQV-REQUIMTE for her contract, under the reference LA/P/0008/2020.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Asogwa, I.S.; Ibrahim, A.N.; Agbaka, J.I. African baobab: Its role in enhancing nutrition, health, and the environment. Trees For. People 2021, 3, 100043. [Google Scholar] [CrossRef]
- Silva, M.L.; Rita, K.; Bernardo, M.A.; Mesquita, M.F.; Pintão, A.M.; Moncada, M. Adansonia digitata L. (Baobab) bioactive compounds, biological activities, and the potential effect on glycemia: A narrative review. Nutrients 2023, 15, 2170. [Google Scholar] [CrossRef]
- Owolodun, B.; Merten, S. Food security from the forest: The case of the commodification of baobab fruit (Adansonia digitata L.) in Boundou region, Senegal. Land 2023, 12, 1423. [Google Scholar] [CrossRef]
- Rana, H.; Kumar, R.; Chopra, A.; Pundir, S.; Gautam, G.K.; Kumar, G. The various pharmacological activity of Adansonia digitata. Res. J. Pharmacol. Pharmacodyn. 2022, 14, 53–59. [Google Scholar] [CrossRef]
- Ismail, B.B.; Pu, Y.; Guo, M.; Ma, X.; Liu, D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chem. 2019, 277, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Badawy, M.T.; Bakr, A.F.; Hegazi, N.M.; Abdellatif, A.; Farag, M.A. Metabolome-based profiling of African baobab fruit (Adansonia digitata L.) using a multiplex approach of MS and NMR techniques in relation to its biological activity. RSC Adv. 2021, 11, 39680–39695. [Google Scholar] [CrossRef] [PubMed]
- Badejo, A.A.; Duyilemi, T.I.; Falarunu, A.J.; Akande, O.A. Inclusion of baobab (Adansonia digitata L.) fruit powder enhances the mineral composition and antioxidative potential of orocessed tigernut (Cyperus esculentus) beverages. Prev. Nutr. Food Sci. 2020, 25, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Evang, E.C.; Habte, T.Y.; Owino, W.O.; Krawinkel, M.B. Can the supplementary consumption of baobab (Adansonia digitata L.) fruit pulp improve the hemoglobin levels and iron status of schoolchildren in Kenya? Findings of a randomized controlled intervention trial. Eur. J. Nutr. 2021, 60, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, M.F.; Tagliamonte, S.; Visconti, A.; Ferracane, R.; Mustafa, A.; Vitaglione, P. Baobab-fruit shell and fibrous filaments are sources of antioxidant dietary fibers. Molecules 2022, 27, 5563. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Gu, L. Occurrence, and biological significance of proanthocyanidins in the American diet. Phytochemistry 2005, 66, 2264–2280. [Google Scholar] [CrossRef]
- Dossa, S.; Dragomir, C.; Plustea, L.; Dinulescu, C.; Cocan, I.; Negrea, M.; Berbecea, A.; Alexa, E.; Rivis, A. Gluten-free cookies enriched with baobab flour (Adansonia digitata L.) and buckwheat flour (Fagopyrum esculentum). Appl. Sci. 2023, 13, 12908. [Google Scholar] [CrossRef]
- Barakat, H. Nutritional and rheological characteristics of composite flour substituted with baobab (Adansonia digitata L.) pulp flour for cake manufacturing and organoleptic properties of their prepared cakes. Foods 2021, 10, 716. [Google Scholar] [CrossRef]
- Darr, D.; Chopi-Msadala, C.; Namakhwa, C.D.; Meinhold, K.; Munthali, C. Processed baobab (Adansonia digitata L.) food products in Malawi: From poor men’s to premium-priced specialty food? Forests 2020, 11, 698. [Google Scholar] [CrossRef]
- Monteiro, S.; Reboredo, F.H.; Lageiro, M.M.; Lourenço, V.M.; Dias, J.; Lidon, F.; Abreu, M.; Martins, A.P.L.; Alvarenga, N. Nutritional of baobab pulp from different angolan origins. Plants 2022, 11, 2272. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Lamien, C.E.; Compaoré, M.M.Y.; Meda, R.N.T.; Kiendrebeogo, M.; Zeba, B.; Millogo, J.F.; Nacoulma, O.G. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 2008, 13, 581–594. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Owoyele, B.V.; Bakare, A.O. Analgesic properties of aqueous bark extract of Adansonia digitata in Wistar rats. Biomed. Pharmacother. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Braca, A.; Sinisgalli, C.; De Leo, M.; Muscatello, B.; Cioni, P.L.; Milella, L.; Ostuni, A.; Giani, S.; Sanogo, R. Phytochemical profile, antioxidant, and antidiabetic activities of Adansonia digitata L. (Baobab) from Mali, as a source of health-promoting compounds. Molecules 2018, 23, 3104. [Google Scholar] [CrossRef]
- El Yahyaoui, O.; Bouabid, B.; Ait Ouaaziz, N.; El Bakkali, M.; El Harche, H.; Lrhorfi, L.A.; Nakari, K.; Bengueddour, R. The antibacterial activity and biochemical composition of Adansonia digitata edible parts. Arab Gulf J. Sci. Res. 2023, 41, 91–106. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubic, N.; Rastija, V.; Agic, D.; Karnaš, M.; Šubaric, D.; Lucic, B. Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative stress and antioxidants—A critical review on in vitro antioxidant assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, S.; Idris, Y.M.A.; Mustafa, S.; Kabeir, B.; Abas, F.; Maullidiani, M.; Abdul Hamid, N. Phytochemical profile and biological activities of Sudanese baobab (Adansonia digitata L.) fruit pulp extract. Int. Food Res. J. 2021, 28, 31–43. [Google Scholar] [CrossRef]
- Stadlmayr, B.; Wanangwe, J.; Waruhiu, C.G.; Jamnadass, R.; Kehlenbeck, K. Nutritional composition of baobab (Adansonia digitata L.) fruit pulp sampled at different geographical locations in Kenya. J. Food Compost. Anal. 2020, 94, 103617. [Google Scholar] [CrossRef]
- Satti, N.M.E. Sudanese baobab (Adansonia digitata). J. North. Basic. Appl. Sci. 2018, 3, 131–147. [Google Scholar]
- Chepngeno, J.; Imathiu, S.; Owino, W.O.; Morlock, G.E. Baobab pulp authenticity and quality control by multi-Imaging high-performance thin-layer chromatography. Food Chem. 2022, 390, 133108. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Silva, A.M.; Santos, C.M.; Pinto, D.C.; Cavaleiro, J.A.; Lima, J.L. 2-Styrylchromones: Novel strong scavengers of reactive oxygen and nitrogen species. Bioorg. Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- European Union. 1169 Regulation (EU) No 1169/2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, L304, 18–63. [Google Scholar]
- Alves, R.C.; Casal, S.; Oliveira, M.B.P.P. Determination of vitamin E in coffee beans by HPLC using a micro-extraction method. Food Sci. Technol. Int. 2009, 15, 57–63. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Vinha, A.F.; Costa, A.S.G.; Barreira, J.C.M.; Pacheco, R.; Oliveira, M.B.P.P. Chemical and antioxidant profiles of acorn tissues from Quercus spp: Potential as new industrial raw materials. Ind. Crops Prod. 2016, 94, 143–151. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Barreira, J.C.M.; Ruas, A.; Vinha, A.F.; Pimentel, F.B.; Alves, R.C.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Improving bioactive compounds extractability of Amorphophallus paeoniifolius (Dennst.) Nicolson. Ind. Crops Prod. 2016, 79, 180–187. [Google Scholar] [CrossRef]
- Rodrigues da Silva, L.; Campos Chisté, R.; Fernandes, E. Chemical and antioxidant characterization of the Portuguese heather honey from Calluna vulgaris. Separations 2021, 8, 177. [Google Scholar] [CrossRef]
- Boeing, J.S.; Ribeiro, D.; Chisté, R.C.; Visentainer, J.V.; Costa, V.M.; Freitas, M.; Fernandes, E. Chemical characterization and protective effect of the Bactris setosa Mart. fruit against oxidative/nitrosative stress. Food Chem. 2017, 220, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.; Chisté, R.C.; Freitas, M.; da Silva, A.F.; Visentainer, J.V.; Fernandes, E. Psidium cattleianum fruit extracts are efficient in vitro scavengers of physiologically relevant reactive oxygen and nitrogen species. Food Chem. 2014, 165, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Muthai, K.U.; Karori, M.S.; Muchugi, A.; Indieka, A.S.; Dembele, C.; Mngomba, S.; Jamnadass, R. Nutritional variation in baobab (Adansonia digitata L.) fruit pulp and seeds based on Africa geographical regions. Food Sci. Nutr. 2017, 5, 1116–1129. [Google Scholar] [CrossRef]
- Chabite, I.T.; Maluleque, I.F.; Cossa, V.J.; Presse, I.J.; Mazuze, I.; Abdula, R.A.; Joaquim, F. Morphological characterization, nutritional and biochemical properties of baobab (Adansonia digitata L.) fruit pulp from two districts of Mozambique. EC Nutr. 2019, 14, 158. [Google Scholar]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.S.H.; Weickert, M.O. The health benefits of dietary fiber. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- OJEU. Commission Decision of authorizing the placing on the market of baobab dried fruit pulp as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (notified under document number C (2008/575/EC). Off. J. Eur. Union. 2008, L183, 38–39. [Google Scholar]
- Wafar, R.J.; Ojinnaka, P.E.; Ndubuisi, D.I. Proximate, anti-nutritional and ascorbic acid composition of baobab fruit pulp meal and its efficiency in ameliorating the impact of heat stress on growth and blood indices of broiler chicks. Acta Sci. Vet. Sci. 2022, 4, 175–184. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for vitamin E as α-tocopherol. EFSA J. 2015, 13, 4149. [Google Scholar] [CrossRef]
- Tembo, D.T.; Holmes, M.J.; Marshall, L.J. Effect of thermal treatment and storage on bioactive compounds, organic acids and antioxidant activity of baobab fruit (Adansonia digitata) pulp from Malawi. J. Food Comp. Anal. 2017, 58, 40–51. [Google Scholar] [CrossRef]
- Ebraheem, S.E.; Idris, Y.M.; Mustafa, S.E.; Baraka, B.M. Identification of flavonoids, phenolics profile by LC-MS/MS and antioxidant activity of crude extracts of baobab (Adansonia digitata. L) fruit pulp from different regions in Sudan. Austin J. Nutr. Food Sci. 2020, 8, 1147. [Google Scholar]
- Ibrahima, C.; Didier, M.; Max, R.; Pascal, D.; Benjamim, Y.; Renaud, B. Biochemical and nutritional properties of baobab pulp from endemic species of Madagascar and the African mainland. Afr. J. Agric. 2013, 8, 6046–6054. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Wasihun, A.A.; Sbhatu, D.B.; Berhe, G.G.; Abay, K.H.; Gebreyohannes, G. Phytochemical constituents of Adansonia digitata L. (Baobab) fruit pulp from Tekeze Valley, Tigrai, Ethiopia. Int. J. Anal. Chem. 2023, 2023, 5591059. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M. An updated review of Adansonia digitata: A commercially important African tree. S. Afr. J. Bot. 2011, 77, 908–919. [Google Scholar] [CrossRef]
- Cancemi, G.; Cicero, N.; Allegra, A.; Gangemi, S. Effect of diet and oxidative stress in the pathogenesis of lymphoproliferative disorders. Antioxidants 2023, 12, 1674. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Yokomizo, A.; Moriwaki, M. Effects of uptake of flavonoids on oxidative stress induced by hydrogen peroxide in human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2006, 70, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Lima, J.L.; Fernandes, E. Optical probes for detection and quantification of neutrophils’ oxidative burst. A review. Anal. Chim. Acta 2009, 649, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Rita, K.; Bernardo, M.A.; Silva, M.L.; Brito, J.; Mesquita, M.F.; Pintão, A.M.; Moncada, M. Adansonia digitata L. (baobab fruit) Effect on postprandial glycemia in healthy adults: A randomized controlled trial. Nutrients 2022, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Ntchapda, F.; Bonabe, C.; Atsamo, A.D.; Azambou, D.R.K.; Fouda, Y.B.; Djibrine, S.I.; Etet, P.F.S.; Théophile, D. Effect of aqueous extract of Adansonia digitata stem bark on the development of hypertension in L-NAME-Induced hypertensive rat model. Evid. Based Complement. Alternat Med. 2020, 2020, 3678469. [Google Scholar] [CrossRef]
- Ibrahim, A.Y.; Mahmoud, M.G.; Asker, M.M.S. Anti-inflammatory, and antioxidant activities of polysaccharide from Adansonia digitata: An in vitro study. Int. J. Pharm. Sci. Rev. Res. 2014, 25, 174–182. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).