It Is Not All about Alkaloids—Overlooked Secondary Constituents in Roots and Rhizomes of Gelsemium sempervirens (L.) J.St.-Hil
Abstract
1. Introduction
2. Results
2.1. Estimation of Total Phenolic Contents by Folin–Ciocalteu Assay
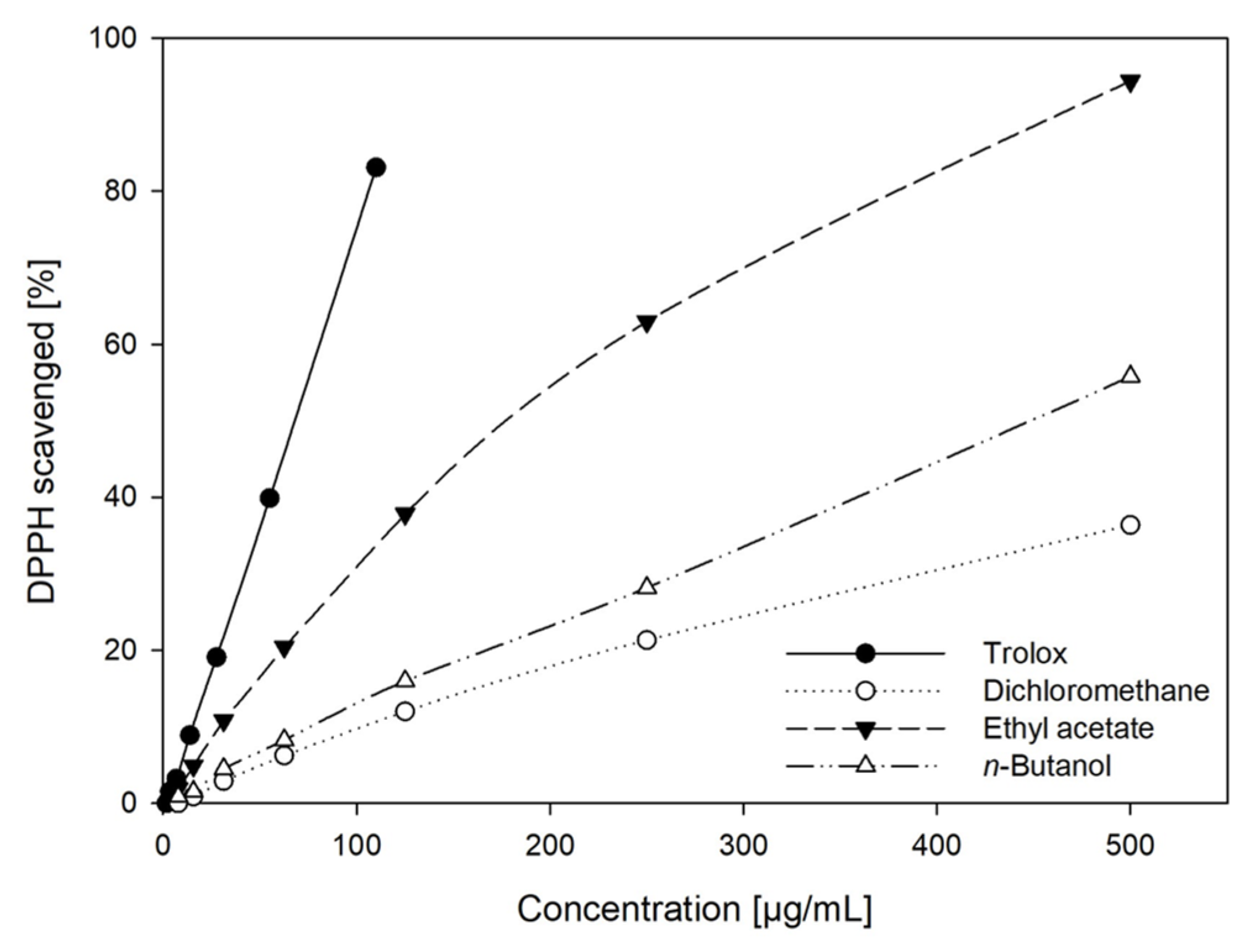
2.2. DPPH Radical Scavenging Activity of GS Extracts
2.3. Analysis of Low Molecular Constituents by GC-MS
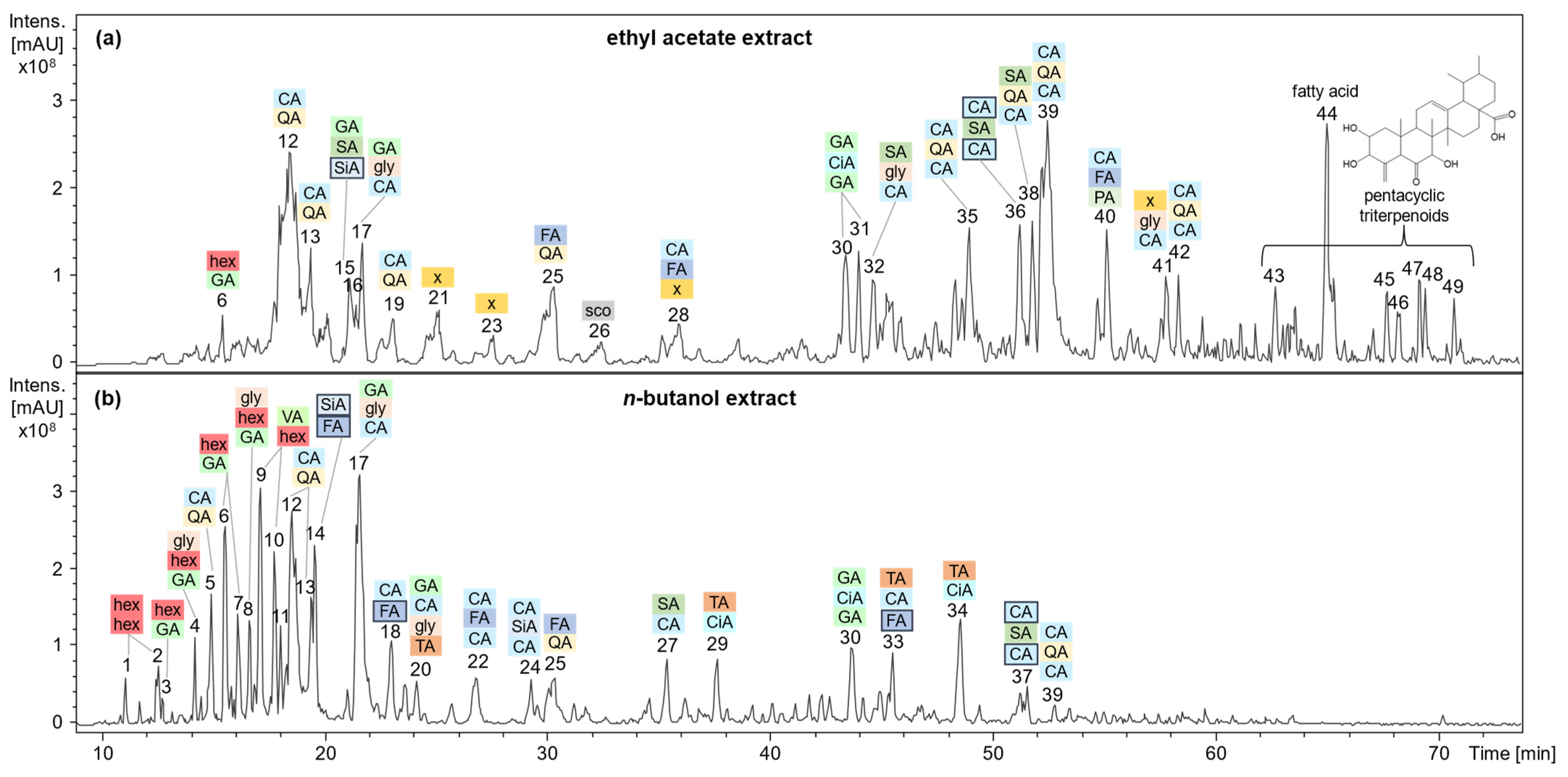
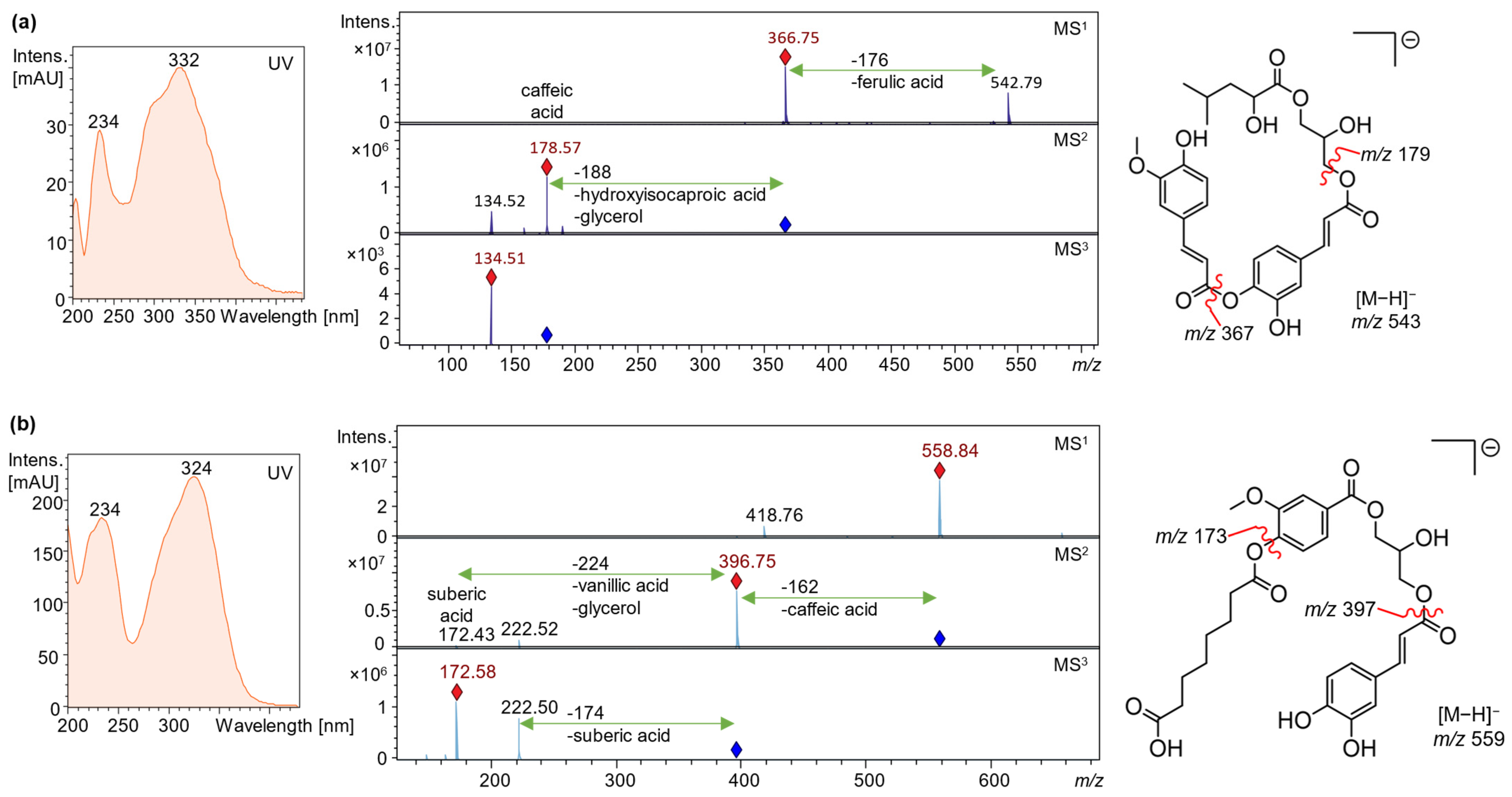
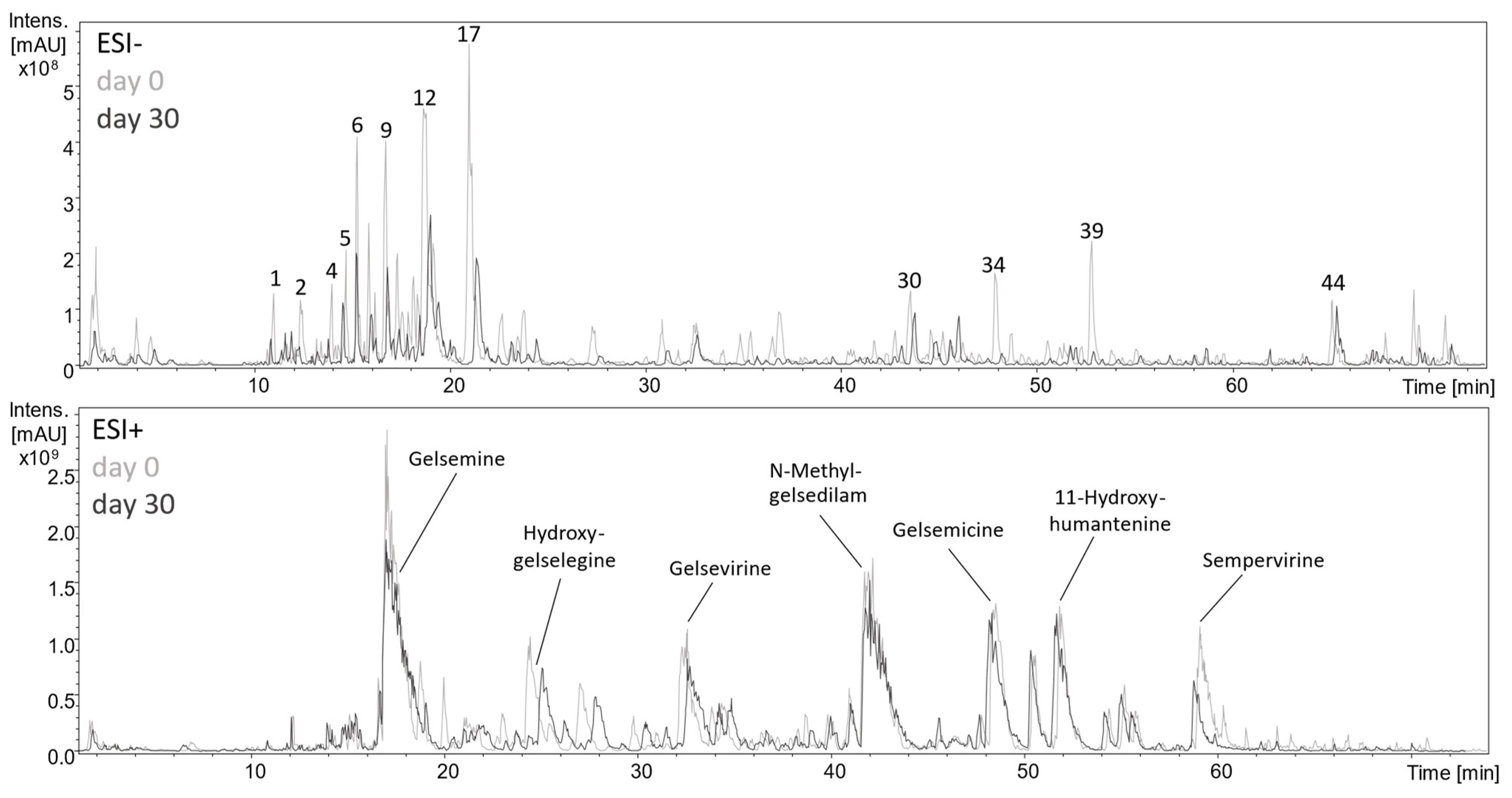
2.4. Analysis of Polar Secondary Constituents by HPLC-DAD-MSn
2.5. Metabolism of Phenolic Constituents upon Lactic Acid Fermentation
3. Discussion
3.1. Phenolic Content and Antioxidant Activity
3.2. Phytochemical and Bioactivity Profiling of G. sempervirens Roots and Rhizomes
3.3. Fermentation of Aqueous GS Root Extracts
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extraction
4.3. Fermentation Experiments
4.4. Estimation of the Total Phenolic Content by Folin–Ciocalteu Assay
4.5. Determination of the DPPH Radical Scavenging Activity
4.6. GC-MS Analyses
4.7. HPLC-DAD-ESI-MSn Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutt, V.; Thakur, S.; Dhar, V.J.; Sharma, A. The genus Gelsemium: An update. Pharmacogn. Rev. 2010, 4, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-L.; Su, Y.-P.; Liu, M.; Xu, Y.; Yang, J.; Liao, K.-J.; Yu, C.-X. Medicinal plants of the genus Gelsemium (Gelsemiaceae, Gentianales)—A review of their phytochemistry, pharmacology, toxicology and traditional use. J. Ethnopharmacol. 2014, 152, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Qiu, H.; Cheng, Y.; Liu, M.; Chen, M.; Que, Y.; Que, W. Gelsemium elegans Benth: Chemical components, pharmacological effects, and toxicity mechanisms. Molecules 2021, 26, 7145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Zhang, C.; Sun, X.; Liao, X.; Zheng, W.; Yin, Q.; Yang, J.; Mao, D.; Wang, B.; et al. The qualitative and quantitative analyses of Gelsemium elegans. J. Pharm. Biomed. Anal. 2019, 172, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, P.; Yuan, W.; Li, S. Steroids, alkaloids, and coumarins from Gelsemium sempervirens. Planta Med. 2008, 74, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.R.; Kirk, O.; Nielsen, B.J.; Norrestam, R. 9-hydroxy substituted iridoids from Gelsemium sempervirens. Phytochemistry 1987, 26, 1725–1731. [Google Scholar] [CrossRef]
- Schun, Y.; Cordell, G.A. Cytotoxic steroids of Gelsemium sempervirens. J. Nat. Prod. 1987, 50, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Lin, L.; Cheng, P.; Sun, Z.-L.; Wu, Y.; Liu, Z.-Y. Fingerprint analysis of Gelsemium elegans by HPLC followed by the targeted identification of chemical constituents using HPLC coupled with quadrupole-time-of-flight mass spectrometry. Fitoterapia 2017, 121, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Xiao, S.; Yang, K.; Ling, L.; Sun, Z.-L.; Liu, Z.-Y. Comprehensive identification and structural characterization of target components from Gelsemium elegans by high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry based on accurate mass databases combined with MS/MS spectra. J. Mass Spectrom. 2017, 52, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Wang, Y.-X. Gelsemium analgesia and the spinal glycine receptor/allopregnanolone pathway. Fitoterapia 2015, 100, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-B.; Tang, M.-H.; Long, J.-Y.; Wang, W.-W.; Qin, J.-Y.; Qi, X.-J.; Liu, Z.-Y. Antinociceptive effect of gelsenicine, principal toxic alkaloids of Gelsemium, on prostaglandin E2-induced hyperalgesia in mice: Comparison with gelsemine and koumine. Biochem. Biophys. Res. Commun. 2023, 681, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, R.M.; Zhang, J.-Y.; Mao, X.-F.; Wang, Y.-X. Gelsemine and koumine, principal active ingredients of Gelsemium, exhibit mechanical antiallodynia via spinal glycine receptor activation-induced allopregnanolone biosynthesis. Biochem. Pharmacol. 2019, 161, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Dutt, V.; Dhar, V.J.; Sharma, A. Antianxiety activity of Gelsemium sempervirens. Pharm. Biol. 2010, 48, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Fungal depsides-naturally inspiring molecules: Biosynthesis, structural characterization, and biological activities. Metabolites 2021, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen depsides and tridepsides: Progress in pharmacological approaches. J. Fungi 2023, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Corrêa, R.C.G.; Barros, L.; Dias, M.I.; Calhelha, R.C.; Correa, V.G.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Effects of in vitro gastrointestinal digestion and colonic fermentation on a rosemary (Rosmarinus officinalis L.) extract rich in rosmarinic acid. Food Chem. 2019, 271, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Landete, J.M.; Rivas, B.d.l.; Muñoz, R. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 2008, 107, 1393–1398. [Google Scholar] [CrossRef]
- Landete, J.M.; Curiel, J.A.; Rodríguez, H.; de Las Rivas, B.; Muñoz, R. Study of the inhibitory activity of phenolic compounds found in olive products and their degradation by Lactobacillus plantarum strains. Food Chem. 2008, 107, 320–326. [Google Scholar] [CrossRef]
- Landete, J.M.; Plaza-Vinuesa, L.; Montenegro, C.; Santamaría, L.; Reverón, I.; de Las Rivas, B.; Muñoz, R. The use of Lactobacillus plantarum esterase genes: A biotechnological strategy to increase the bioavailability of dietary phenolic compounds in lactic acid bacteria. Int. J. Food Sci. Nutr. 2021, 72, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Singh, D.P.; Govindarajan, R.; Khare, A.; Rawat, A.K.S. Optimization of a high-performance liquid chromatography method for the separation and identification of six different classes of phenolics. J. Chromatogr. Sci. 2007, 45, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Tasfiyati, A.N.; Antika, L.D.; Septama, A.W.; Hikmat, H.; Kurniawan, H.H.; Ariani, N. A validated HPLC-DAD method and comparison of different extraction techniques for analysis of scopoletin in noni-based products. Kuwait J. Sci. 2023, 50, 276–281. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC-DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Comp. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Bunse, M.; Mailänder, L.K.; Lorenz, P.; Stintzing, F.C.; Kammerer, D.R. Evaluation of Geum urbanum L. extracts with respect to their antimicrobial potential. Chem. Biodivers. 2022, 19, e202100850. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Jin, J.; Lao, J.; Zhou, R.; He, W.; Qin, Y.; Zhong, C.; Xie, J.; Liu, H.; Wan, D.; Zhang, S.; et al. Simultaneous identification and dynamic analysis of saccharides during steam processing of rhizomes of Polygonatum cyrtonema by HPLC-QTOF-MS/MS. Molecules 2018, 23, 2855. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bertrams, J.; Müller, M.B.; Kunz, N.; Kammerer, D.R.; Stintzing, F.C. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J. Appl. Bot. Food Qual. 2013, 86, 143–153. [Google Scholar] [CrossRef]
- Lorenz, P.; Knittel, D.N.; Conrad, J.; Lotter, E.M.; Heilmann, J.; Stintzing, F.C.; Kammerer, D.R. 1-Acetyl-3-(3R)-hydroxyfatty acylglycerols: Lipid Compounds from Euphrasia rostkoviana Hayne and E. tetraquetra (Bréb.) Arrond. Chem. Biodivers. 2016, 13, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-R.; He, X.-F.; Jin, X.-J.; Pan, H.; Shi, Z.-N.; Xu, D.-D.; Yao, X.-J.; Zhu, Y. New nor-ursane type triterpenoids from Gelsemium elegans. Fitoterapia 2015, 106, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Conrad, J.; Stintzing, F.C. Metabolic fate of depsides and alkaloid constituents in aqueous extracts from Mercurialis perennis L. during fermentation. Chem. Biodivers. 2013, 10, 1706–1723. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Schneider, S.; Stintzing, F.C.; Carle, R.; Reichling, J. Efficacy of an aqueous Pelargonium sidoides extract against herpesvirus. Phytomedicine 2008, 15, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How does the phenol structure influence the results of the Folin-Ciocalteu assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the Folin-Ciocalteu method for the estimation of (poly)phenol content in food: Total phenolic intake in a mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Kyoung Chun, O.; Kim, D.-O. Consideration on equivalent chemicals in total phenolic assay of chlorogenic acid-rich plums. Food Res. Int. 2004, 37, 337–342. [Google Scholar] [CrossRef]
- White, L.M. Carbohydrate reserves of grasses: A review. J. Range Manag. 1973, 26, 13–18. [Google Scholar] [CrossRef]
- Peng, Y.-L.; Liang, J.-J.; Xue, Y.; Khan, A.; Zhang, P.-P.; Feng, T.-T.; Song, D.; Zhou, Y.; Wei, X. Genus Gelsemium and its endophytic fungi—Comprehensive review of their traditional uses, phytochemistry, pharmacology, and toxicology. Curr. Top. Med. Chem. 2023, 23, 2452–2487. [Google Scholar] [CrossRef] [PubMed]
- Obi Johnson, B.; Golonka, A.M.; Blackwell, A.; Vazquez, I.; Wolfram, N. Floral scent variation in the heterostylous species Gelsemium sempervirens. Molecules 2019, 24, 2818. [Google Scholar] [CrossRef]
- Chen, L.; Wei, X.; Matsuda, Y. Depside bond formation by the starter-unit acyltransferase domain of a fungal polyketide synthase. J. Am. Chem. Soc. 2022, 144, 19225–19230. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, H.; Sohrabi, M.; Yousefi, M.; Boustie, J. Tridepsides as potential bioactives: A review on their chemistry and the global distribution of their lichenic and non-lichenic natural sources. Front. Fungal Biol. 2023, 4, 1088966. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.M.; Szopa, A.; Kubica, P. High production of depsides and other phenolic acids in different types of shoot cultures of three Aronias: Aronia melanocarpa, Aronia arbutifolia, Aronia × prunifolia. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications, 1st ed.; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer International Publishing; Imprint Springer: Cham, Switzerland, 2021; pp. 337–364. ISBN 978-3-030-30184-2. [Google Scholar]
- Jin, Q.; Hu, X.; Deng, Y.; Hou, J.; Lei, M.; Ji, H.; Zhou, J.; Qu, H.; Wu, W.; Guo, D. Four new depsides isolated from Salvia miltiorrhiza and their significant nerve-protective activities. Molecules 2018, 23, 3274. [Google Scholar] [CrossRef] [PubMed]
- Steingass, C.B.; Glock, M.P.; Schweiggert, R.M.; Carle, R. Studies into the phenolic patterns of different tissues of pineapple (Ananas comosus L. Merr.) infructescence by HPLC-DAD-ESI-MS(n) and GC-MS analysis. Anal. Bioanal. Chem. 2015, 407, 6463–6479. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, H.; Sashida, Y.; Mimaki, Y. Phenolic glycerides from Lilium auratum. Phytochemistry 1987, 26, 844–845. [Google Scholar] [CrossRef]
- Delaporte, R.H.; Guzen, K.P.; Laverde, A.; dos Santos, A.R.; Sarragiotto, M.H. Phenylpropanoid glycerols from Tillandsia streptocarpa Baker (Bromeliaceae). Biochem. Syst. Ecol. 2006, 34, 599–602. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC-LTQ/Orbitrap/MS/MS) study of phenolic profile of Serbian poplar type propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Guzelmeric, E.; Yuksel, P.I.; Yaman, B.K.; Sipahi, H.; Celik, C.; Kırmızıbekmez, H.; Aydın, A.; Yesilada, E. Comparison of antioxidant and anti-inflammatory activity profiles of various chemically characterized Turkish propolis sub-types: Which propolis type is a promising source for pharmaceutical product development? J. Pharm. Biomed. Anal. 2021, 203, 114196. [Google Scholar] [CrossRef] [PubMed]
- Okińczyc, P.; Widelski, J.; Szperlik, J.; Żuk, M.; Mroczek, T.; Skalicka-Woźniak, K.; Sakipova, Z.; Widelska, G.; Kuś, P.M. Impact of plant origin on Eurasian propolis on phenolic profile and classical antioxidant activity. Biomolecules 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Thuong, P.T.; Hwang, I.H.; Bae, K.; Kim, B.Y.; Osada, H.; Ahn, J.S. Protein tyrosine phosphatase 1B inhibitory activity of 24-norursane triterpenes isolated from Weigela subsessilis. Phytother. Res. 2010, 24, 1716–1719. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Ma, S.-G.; Zhang, D.; Li, Y.-H.; Qu, J.; Li, Y.; Liu, Y.-B.; Yu, S.-S. Oxygenated pentacyclic triterpenoids from the stems and branches of Enkianthus chinensis. Bioorg. Chem. 2021, 111, 104866. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-D.; He, J.; Li, X.-Y.; Dong, L.-B.; Gong, X.; Gao, X.; Song, L.-D.; Li, Y.; Peng, L.-Y.; Zhao, Q.-S. Triterpenoids and steroids with cytotoxic activity from Emmenopterys henryi. Planta Med. 2013, 79, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-J.; Pu, J.; Zhang, H.; Han, T.; Rahman, K.; Qin, L.-P. Sesquiterpenoids and norterpenoids from Vitex negundo. Fitoterapia 2012, 83, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, L.; Reverón, I.; de Felipe, F.L.; de Las Rivas, B.; Muñoz, R. Ethylphenol formation by Lactobacillus plantarum: Identification of the enzyme involved in the reduction of vinylphenols. Appl. Environ. Microbiol. 2018, 84, e01064-18. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.; Schieberle, P. Characterization of the most odor-active compounds in an American Bourbon whisky by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008, 56, 5813–5819. [Google Scholar] [CrossRef] [PubMed]
- de Ovalle, S.; Brena, B.; González-Pombo, P. Influence of beta glucosidases from native yeast on the aroma of Muscat and Tannat wines. Food Chem. 2021, 346, 128899. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Torres, M.; Mancheño, J.M.; de Las Rivas, B.; Muñoz, R. Characterization of a cold-active esterase from Lactobacillus plantarum suitable for food fermentations. J. Agric. Food Chem. 2014, 62, 5126–5132. [Google Scholar] [CrossRef] [PubMed]
- Mailänder, L.K.; Lorenz, P.; Bitterling, H.; Stintzing, F.C.; Daniels, R.; Kammerer, D.R. Phytochemical characterization of chamomile (Matricaria recutita L.) roots and evaluation of their antioxidant and antibacterial potential. Molecules 2022, 27, 8508. [Google Scholar] [CrossRef] [PubMed]
- Que, W.; Chen, M.; Yang, L.; Zhang, B.; Zhao, Z.; Liu, M.; Cheng, Y.; Qiu, H. A network pharmacology-based investigation on the bioactive ingredients and molecular mechanisms of Gelsemium elegans Benth against colorectal cancer. BMC Complement. Med. Ther. 2021, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Kogure, N.; Someya, A.; Urano, A.; Kitajima, M.; Takayama, H. Total synthesis and full NMR assignment of ourouparine, a yohimbane-type indole alkaloid isolated from Gelsemium sempervirens. J. Nat. Med. 2007, 61, 208–212. [Google Scholar] [CrossRef]


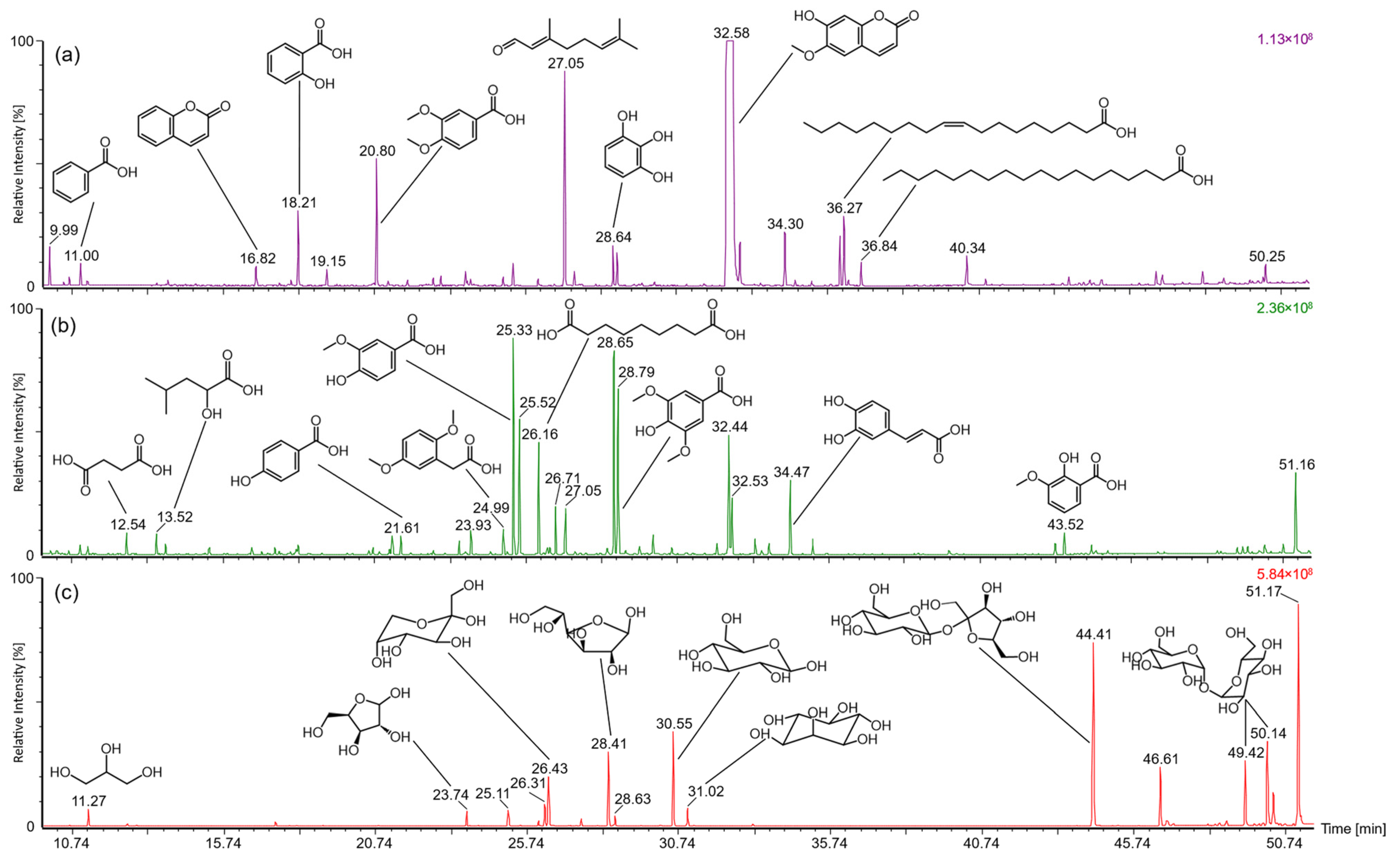


| tR [min] | Constituent (TMS Derivative) | MW [Da] | Fragment m/z (Intensity %) |
|---|---|---|---|
| (a) Dichloromethane | |||
| 10.0 | 2-Octenoic acid | 214.1 | 214 (38), 199 (82), 124 (100), 109 (62), 73 (88), 55 (28) |
| 11.0 | Benzoic acid | 194.3 | 194 (7), 179 (100), 135 (66), 105 (65), 77 (49) |
| 16.8 | Coumarin | 146.1 | 146 (96), 118 (100), 89 (45), 75 (12), 77 (49) |
| 18.2 | Salicylic acid | 282.5 | 267 (100), 209 (10), 73 (82) |
| 19.2 | Vanillin | 224.3 | 224 (26), 209 (46), 194 (100), 73 (21) |
| 20.8 | Veratric acid | 254.4 | 254 (44), 239 (100), 136 (93), 73 (90) |
| 27.1 | Citral | 152.2 | 152 (53), 107 (26), 84 (73), 69 (100) |
| 28.6 | Pyrogallol | 342.7 | 329 (36), 239 (34), 209 (21), 147 (49), 93 (32), 73 (100) |
| 28.8 | Syringic acid | 342.5 | 342 (67), 327 (100), 312 (79), 297 (63), 253 (39), 73 (71) |
| 32.6 | Scopoletin | 264.3 | 264 (48), 234 (100), 206 (37), 191 (8), 176 (10), 73 (36) |
| 34.3 | Adenine | 279.5 | 294 (37), 279 (25), 264 (100), 73 (30) |
| 36.2 | Linoleic acid | 352.3 | 337 (31), 129 (37), 117 (32), 95 (44), 81 (58), 73 (100), 55 (54) |
| 36.3 | Oleic acid | 354.3 | 354 (2), 339 (51), 129 (68), 117 (91), 73 (100), 55 (63) |
| 36.8 | Stearic acid | 356.3 | 356 (6), 341 (78), 132 (63), 117 (100), 73 (80), 55 |
| 40.3 | Undefined sterol | 440 (4), 369 (8), 225 (75), 130 (23), 93 (17), 73 (100) | |
| 50.2 | Undefined sterol | 386 (7), 371 (37), 281 (36), 269 (46), 207 (42), 73 (100) | |
| (b) Ethyl acetate | |||
| 12.5 | Succinic acid | 262.4 | 247 (11), 147 (100), 73 (35) |
| 13.5 | 2-Hydroxy-isocaproic acid | 276.5 | 247 (66), 159 (82), 147 (47), 115 (19), 73 (100) |
| 21.6 | Salicylic acid | 282.5 | 282 (22), 267 (38), 193 (18), 73 (100) |
| 23.9 | Suberic acid | 318.6 | 303 (58), 213 (30), 147 (71), 73 (100), 69 (44), 55 (66) |
| 25.0 | 2,5-Dimethoxy-phenylacetic acid | 268.4 | 268 (16), 253 (32), 209 (38), 134 (25), 105 (36), 91 (29), 73 (100) |
| 25.3 | Vanillic acid | 312.1 | 312 (57), 297 (100), 282 (34), 267 (67), 253 (51), 223 (51), 126 (31), 73 (49) |
| 25.5 | Gentisic acid | 370.6 | 370 (4), 355 (100), 73 (60) |
| 26.2 | Azelaic acid | 332.6 | 317 (51), 201 (52), 147 (26), 129 (31), 117 (29), 73 (100), 55 (48) |
| 26.7 | Protocatechuic acid | 370.6 | 370 (49), 355 (26), 311 (21), 193 (100), 73 (53) |
| 27.1 | Citral | 152.2 | 152 (42), 107 (23), 93 (23), 84 (67), 81 (29), 75 (60), 69 (100) |
| 28.7 | Pyrogallol | 342.7 | 329 (27), 239 (29), 209 (18), 147 (46), 143 (34), 119 (28), 103 (28), 73 (100) |
| 28.8 | Syringic acid | 342.5 | 342 (66), 327 (100), 312 (76), 297 (66), 253 (43), 149 (32), 141 (33), 73 (77) |
| 32.4 | Scopoletin | 264.3 | 264 (50), 234 (100), 206 (40), 191 (8), 176 (11), 73 (31) |
| 32.5 | Vanillylmandelic acid | 414.7 | 428 (64), 297 (100), 73 (83) |
| 34.5 | Caffeic acid | 396.7 | 396 (71), 381 (19), 219 (100), 191 (16), 73 (79) |
| 43.5 | Methoxysalicylic acid | 312.5 | 297 (100), 73 (48) |
| 51.2 | Saccharide derivative | 331 (30), 253 (85), 217 (100), 204 (21), 147 (31), 103 (25), 93 (27), 73 (89) | |
| (c) n-Butanol | |||
| 11.3 | Glycerol | 308.6 | 218 (19), 205 (58), 147 (90), 133 (20), 117 (33), 103 (31), 73 (100) |
| 23.7 | Xylose | 438.8 | 217 (39), 204 (100), 191 (41), 147 (33), 73 (66) |
| 25.1 | Arabinose | 438.8 | 217 (45), 204 (100), 191 (45), 147 (34), 73 (83) |
| 26.3 | Fructofuranose | 541.1 | 437 (13), 217 (78), 147 (28), 73 (100) |
| 26.4 | Fructopyranose | 541.1 | 437 (24), 217 (27), 204 (78), 147 (37), 73 (100) |
| 28.4 | Glucose | 541.1 | 217 (18), 204 (100), 191 (50), 147 (24), 73 (62) |
| 28.6 | Galactose | 541.1 | 329 (22), 239 (23), 217 (18), 204 (63), 191 (35), 147 (56), 143 (27), 73 (100), |
| 30.6 | Glucopyranose | 541.1 | 217 (19), 204 (100), 191 (54), 147 (24), 73 (62) |
| 31.0 | Myo-Inositol | 613.2 | 318 (23), 305 (32), 217 (70), 191 (27), 147 (48), 133 (33), 73 (100) |
| 44.4 | Sucrose | 919.7 | 437 (18), 361 (100), 217 (43), 147 (25), 103 (19), 73 (81) |
| 46.6 | Unknown | 394.5 | 394 (22), 351 (21), 323 (16), 134 (16), 108 (100), 73 (43) |
| 49.4 | Disaccharide | 919.7 | 361 (94), 340 (38), 251 (38), 217 (33), 204 (20), 191 (28), 147 (31), 73 (100) |
| 50.1 | Disaccharide | 919.7 | 373 (19), 217 (18), 204 (100), 147 (16), 73 (47) |
| 51.2 | Saccharide derivative | 331 (29), 253 (85), 217 (100), 204 (22), 147 (26), 103 (23), 93 (24), 73 (83) | |
| Peak Number | tR [min] | λmax [nm] | Negative Ionization m/z | Compound Assignment | |||
|---|---|---|---|---|---|---|---|
| EtOAc a | BuOH b | MS1 | MS2 | MS3 | |||
| 1 | 11.1 | - | 421 c, 411 | 225, 179 c | 161, 143 c, 119 | Saccharide | |
| 2 | 12.4 | 204 | 393 d | 347 c | 185, 161 c, 143 | Saccharide | |
| 3 | 12.5 | ND e | 375 c | 213 c | 169 c | Gallic acid hexoside | |
| 4 | 14.2 | 206, 230, 308 | 421 c | 179 c | 143 c | Methylgallic acid hexoside glycerol ester | |
| 5 | 14.9 | 204, 324 | 353 c | 191 c, 179 | 173 c | 4-O-Caffeoylquinic acid | |
| 6 | 6 | 15.5 | 238 | 375 c | 213, 169 c | 151, 125 c, 109 | Gallic acid hexoside |
| 7 | 16.1 | 252 | 375 c | 213 c, 169 | 125, 107 c | Gallic acid hexoside | |
| 8 | 16.6 | 208, 254 | 421 c | 179 c | 143 c, 119, 89 | Methylgallic acid hexoside glycerol ester | |
| 9 | 17.1 | 209, 254 | 407 c, 397 | 343 c | 179 c, 161, 143, 119 | Veratric acid hexoside derivative | |
| 10 | 17.7 | 204, 288 | 407 c | 343, 179 c | 143, 119, 89, 83 | Veratric acid hexoside derivative | |
| 11 | 18.0 | 204, 225, 290sh, 328 | 377 d | 331 c | 161 c | Hydroxycoumarin and azelaic acid ester | |
| 12 | 12 | 18.5 | 218, 236, 312, 328 | 707, 353 c | 191 | 173, 127, 111, 93 | 5-O-Caffeoylquinic acid |
| 13 | 13 | 19.4 | 220, 240sh, 290sh, 326 | 353 c | 191, 173 c | 127, 93 | 4-O-Caffeoylquinic acid |
| 14 | 19.6 | 224, 324 | 403 c, 353 | 237, 195 c | 165, 151, 97 | Dihydroferuloyl-dihydrosinapinic acid | |
| 15 | 21.1 | 216, 274 | 557 c | 197 c | 181, 153, 137 c | Syringyl-galloyl-dihydrosinapinic acid | |
| 16 | 21.4 | 222, 296 | 505 c, 405 | 145 c | Succinyl-galloyl-dihydrosinapinic acid derivative | ||
| 17 | 17 | 21.7 | 226, 302 | 405 c | 225, 179 c | 89 c | Gallic and caffeic acid glyceride |
| 18 | 23.0 | 230, 326 | 403 d | 357, 195 c, 179 | 151, 125 c | Caffeoyl-dihydroferulic acid | |
| 19 | 23.1 | 230, 316 | 353 c | 191 c | 171, 127 | 3-O-Caffeoylquinic acid | |
| 20 | 24.1 | 308 | 537 c | 311 c | 293, 233, 149 c, 101 | Caffeoyl-galloyl-tartaric acid glyceride | |
| 21 | 25.1 | 218, 316 | 391 f, 195 c | 151 c | Dihydroferulic or dimethylphenyl-acetic acid | ||
| 22 | 26.8 | 238, 324 | 517 c | 193 c | 176, 149, 134 c | Dicaffeoyl-ferulic acid | |
| 23 | 27.6 | 226, 264 | 363 c | 315 c, 272 | 300, 272, 256 c | Methylellagic acid derivative | |
| 24 | 29.3 | 238, 324 | 547 c | 367, 325, 295, 265, 223 c | 205, 163 c | Dicaffeoyl-sinapinic acid | |
| 25 | 25 | 30.3 | 212, 324 | 367 c | 191 c, 173 | 173 c, 93 | Feruloylquinic acid |
| 26 | 32.4 | 207, 228, 296, 342 | 191 c | 176 c | Scopoletin | ||
| 27 | 35.4 | 308 | 359 c | 197 c | 153 c, 135, 109 | Caffeoylsyringic acid | |
| 28 | 35.9 | 204, 234, 332 | 543, 367 c | 179 c, 161 | 135 c | Feruloyl-caffeoyl- hydroxyisocaproic acid glyceride | |
| 29 | 37.6 | 224, 282 | 467 d | 421 c, 293 | 293 c, 191, 149 | Tartaric and methylcinnamic acid ester | |
| 30 | 30 | 43.5 | 224, 282 | 495 c | 463, 327 c | 311, 183 | Digalloylcinnamic acid derivative |
| 31 | 44.0 | 222, 260sh, 286 | 509 c | 327 c | 183 c | Digalloylcinnamic acid derivative | |
| 32 | 44.6 | 222, 264, 300 | 465 c | 433 c | 289, 271 c, 179 | Caffeoylsyringic acid glyceride | |
| 33 | 45.5 | 228 | 549 d | 503 c | 371 c, 161 | Dihydroferuloyl-caffeoyltartaric acid | |
| 34 | 48.6 | 232 | 481 d | 435 c, 293 | 293 c, 149 | Tartaric and methylcinnamic acid ester | |
| 35 | 49.0 | 222, 235, 300sh, 326 | 515 c | 353 c | 191 c, 179, 135 | 3,5-Di-O-caffeoylquinic acid | |
| 36 | 51.2 | 222, 282 | 539 c, 515 | 523 c, 341 | 197 c | Di-dihydrocaffeoyl-syringic acid methyl ester | |
| 37 | 51.5 | 224, 288 | 541 c | 523 c | 197 c | Di-dihydrocaffeoyl-syringic acid methyl ester derivative | |
| 38 | 51.8 | 232, 292 | 533 c | 371 c | 197, 173 c | Syringyl- caffeoyl-quinic acid | |
| 39 | 39 | 52.5 | 220, 240, 300sh, 326 | 515 c | 353 c | 173 c | 4,5-Di-O-caffeoylquinic acid |
| 40 | 55.1 | 232, 324 | 491 c | 315 c | 153 c | Feruloyl-caffeoyl-protocatechuic acid | |
| 41 | 57.8 | 234, 324 | 559 c | 397 c | 223, 173 c | Caffeoyl-vanillyl-suberic acid glyceride | |
| 42 | 58.4 | 232, 290, 340 | 515 c | 353 c | 191 c, 179, 173 | 3,4-Di-O-caffeoylquinic acid | |
| 43 | 62.7 | 232, 326 | 501 c | 483 c, 465, 439 | 419 c, 403 | Gelse-norursane A | |
| 44 | 65.0 | - | 329 c | 211, 293, 229 c, 171, 158 | Trihydroxy-octadecenoic acid isomer | ||
| 45 | 67.7 | - | 483 c | 419, 391 c, 379, 203 | 321 c | Gelse-norursane derivative | |
| 46 | 68.2 | - | 515 c | 471 c | 453 c, 427 | Gelse-norursane C derivative | |
| 47 | 69.1 | - | 485 c | 467 c | 437 c, 355 | Gelse-norursane B | |
| 48 | 69.4 | - | 485 c | 467, 405 c | 363 c | Gelse-norursane B | |
| 49 | 70.9 | - | 469 c | 405 c | 375 c | Gelse-norursane E | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mailänder, L.K.; Nosrati Gazafroudi, K.; Lorenz, P.; Daniels, R.; Stintzing, F.C.; Kammerer, D.R. It Is Not All about Alkaloids—Overlooked Secondary Constituents in Roots and Rhizomes of Gelsemium sempervirens (L.) J.St.-Hil. Plants 2024, 13, 2208. https://doi.org/10.3390/plants13162208
Mailänder LK, Nosrati Gazafroudi K, Lorenz P, Daniels R, Stintzing FC, Kammerer DR. It Is Not All about Alkaloids—Overlooked Secondary Constituents in Roots and Rhizomes of Gelsemium sempervirens (L.) J.St.-Hil. Plants. 2024; 13(16):2208. https://doi.org/10.3390/plants13162208
Chicago/Turabian StyleMailänder, Lilo K., Khadijeh Nosrati Gazafroudi, Peter Lorenz, Rolf Daniels, Florian C. Stintzing, and Dietmar R. Kammerer. 2024. "It Is Not All about Alkaloids—Overlooked Secondary Constituents in Roots and Rhizomes of Gelsemium sempervirens (L.) J.St.-Hil" Plants 13, no. 16: 2208. https://doi.org/10.3390/plants13162208
APA StyleMailänder, L. K., Nosrati Gazafroudi, K., Lorenz, P., Daniels, R., Stintzing, F. C., & Kammerer, D. R. (2024). It Is Not All about Alkaloids—Overlooked Secondary Constituents in Roots and Rhizomes of Gelsemium sempervirens (L.) J.St.-Hil. Plants, 13(16), 2208. https://doi.org/10.3390/plants13162208







