Long-Term Straw Returning Enhances Phosphorus Uptake by Zea mays L. through Mediating Microbial Biomass Phosphorus Turnover and Root Functional Traits
Abstract
:1. Introduction
2. Results
2.1. Crop Yield and P Uptake
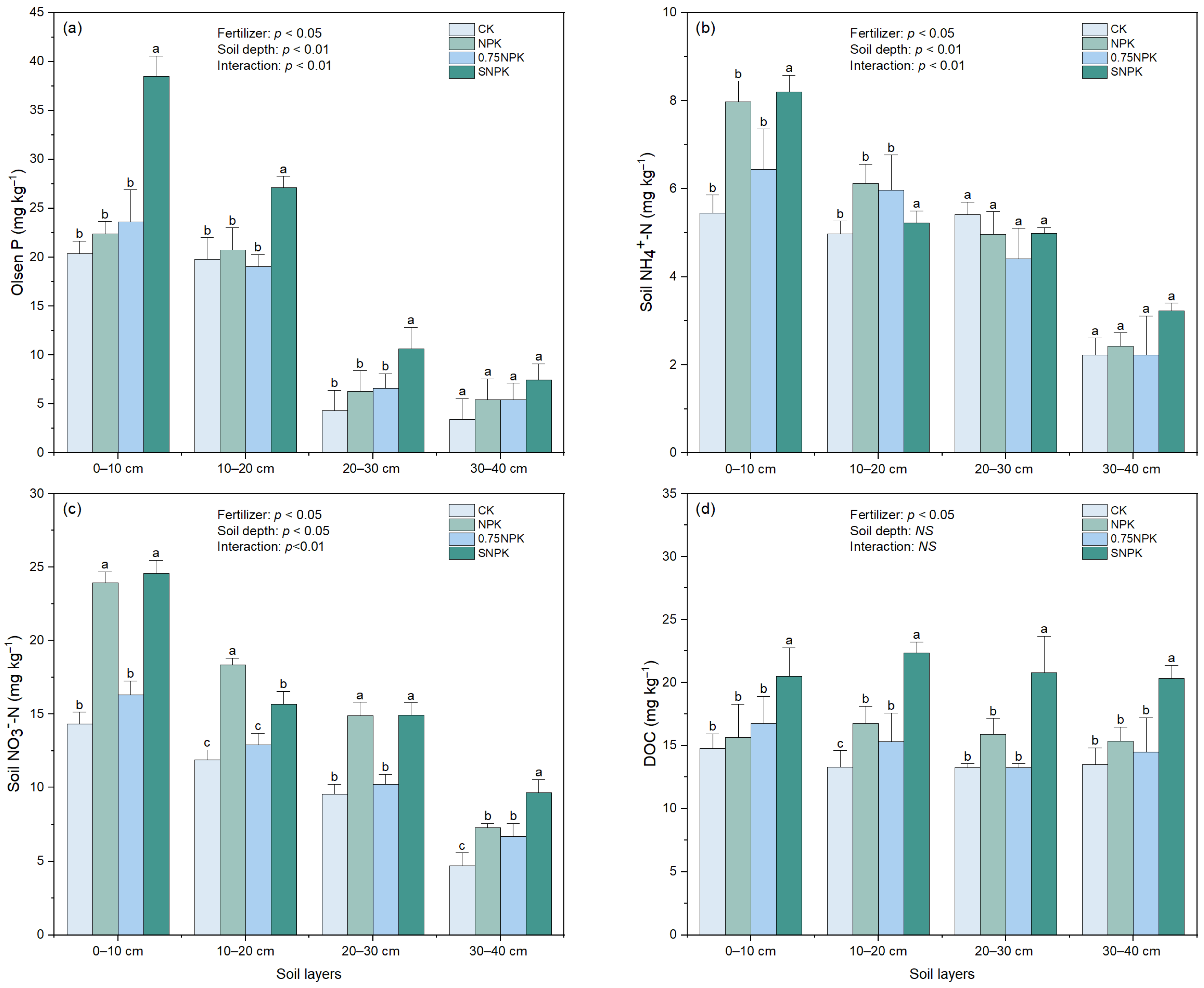
2.2. Soil Nutrient Availability
2.3. Soil P Availability
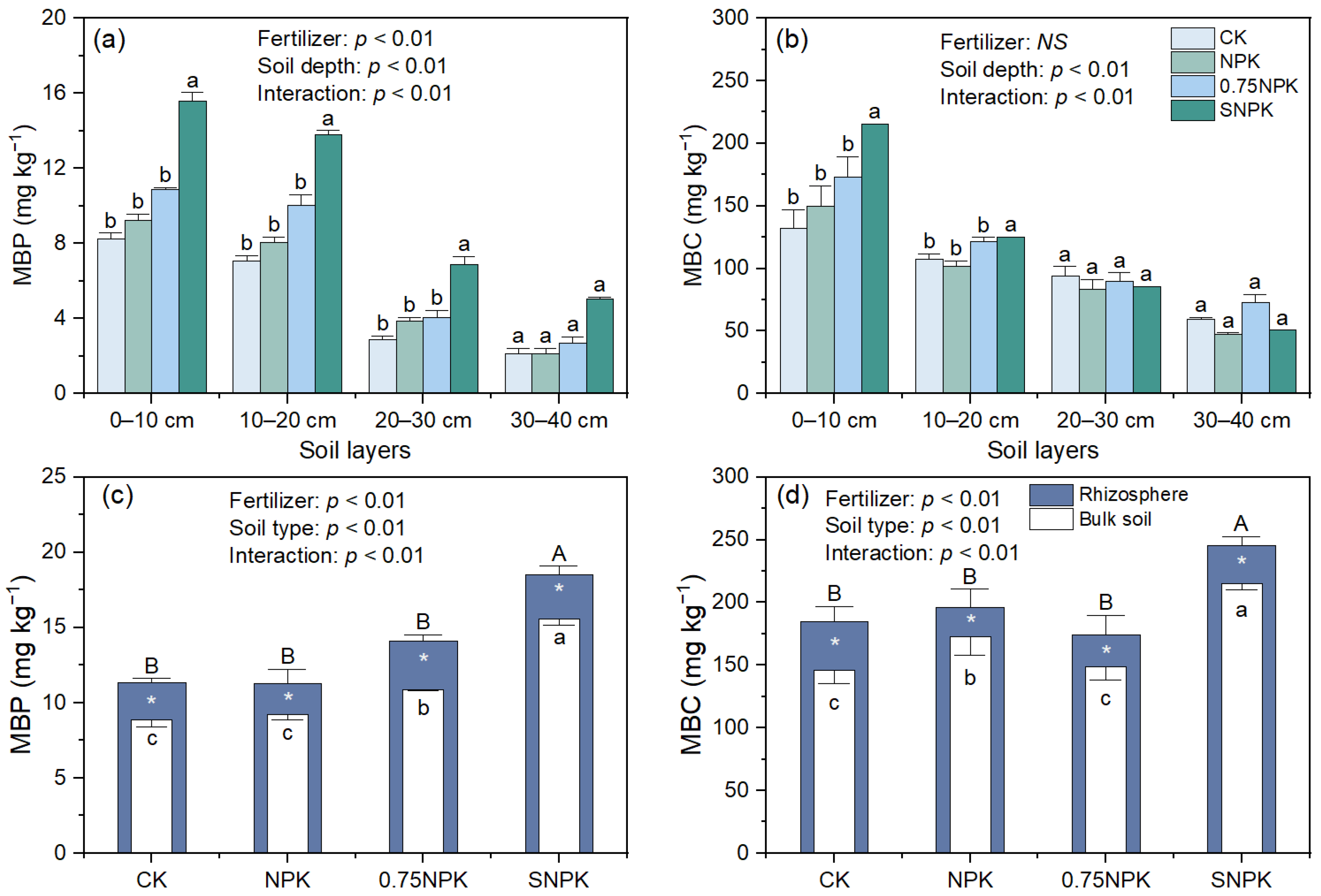
2.4. Microbial Biomass and Functionality
2.5. Root P-Acquisition Strategies
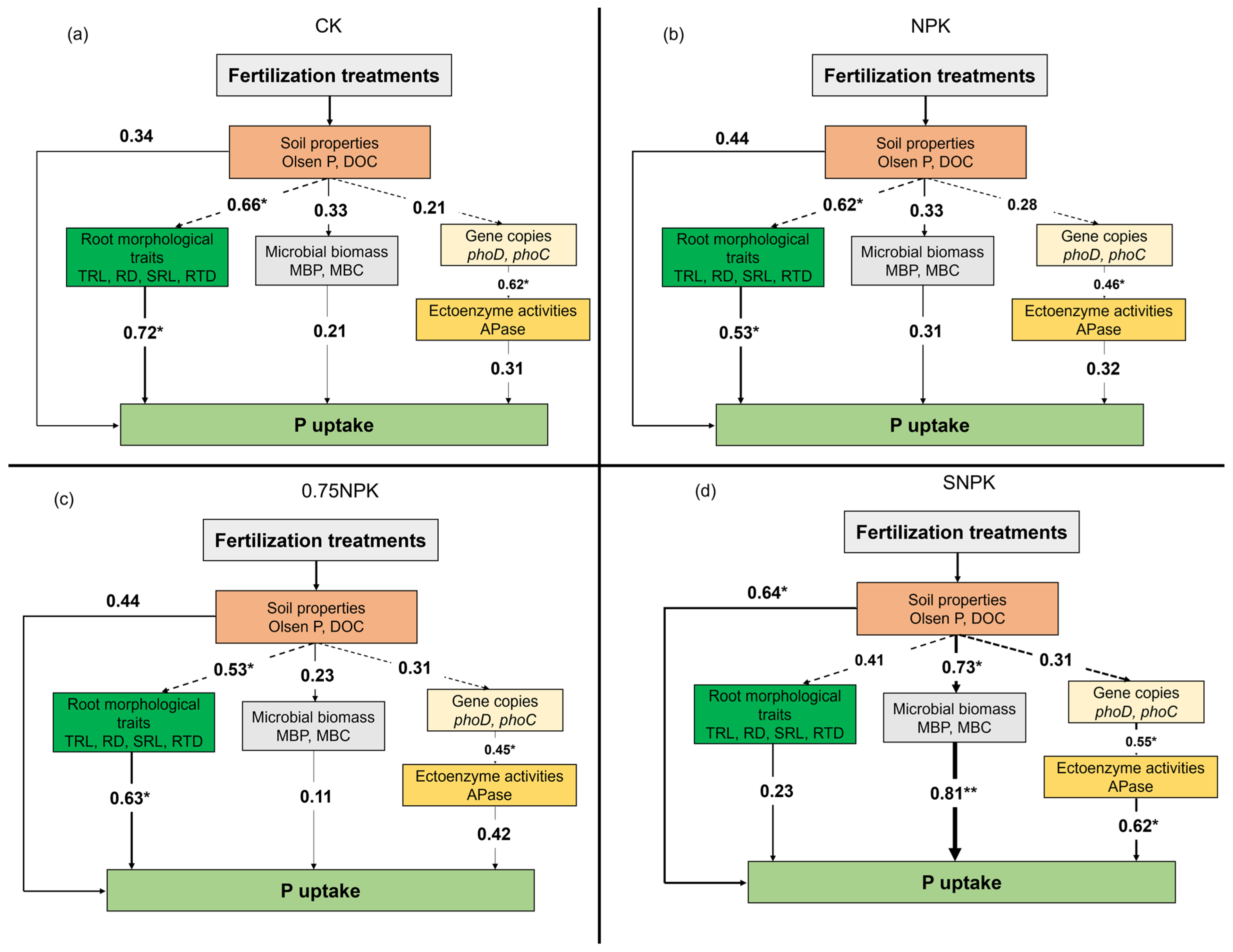
2.6. Drivers of Crop P Uptake under Different Fertilizer Regimes
3. Discussion
3.1. Response of Shoot P Uptake and Soil P Availability to Different Fertilizer Regimes
3.2. Microbial Biomass and Functional Traits Vary among Different Fertilizer Regimes
3.3. Root P-Acquisition Strategies in Response to Different Fertilizer Regimes
3.4. Phosphorus Mobilization Drivers Varied among Fertilizer Treatments
4. Materials and Methods
4.1. Experimental Site
4.2. Experimental Design
4.3. Harvest and Measurements
4.3.1. Plant Sampling and Measurements
4.3.2. Soil and Rhizosphere Sampling and Measurements
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: Implications for China. Adv. Agron. 2010, 107, 1–32. [Google Scholar] [CrossRef]
- Renard, D.; Tilman, D. National food production stabilized by crop diversity. Nature 2019, 571, 257–260. [Google Scholar] [CrossRef]
- Zhou, D.; Su, Y.; Ning, Y.; Rong, G.; Wang, G.; Liu, D.; Liu, L. Estimation of the effects of maize straw return on soil carbon and nutrients using response surface methodology. Pedosphere 2018, 28, 411–421. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, W.; Li, T.; Cheng, X.; Liu, Q. Balancing straw returning and chemical fertilizers in China: Role of straw nutrient resources. Renew. Sust. Energ. Rev. 2018, 81, 2695–2702. [Google Scholar] [CrossRef]
- Robertson, F.A.; Nash, D.M. Phosphorus and nitrogen in soil, plants, and overland flow from sheep-grazed pastures fertilized with different rates of superphosphate. Agric. Ecosyst. Environ. 2008, 126, 195–208. [Google Scholar] [CrossRef]
- Bi, Q.F.; Li, K.J.; Zheng, B.X.; Liu, X.P.; Li, H.Z.; Jin, B.J.; Ding, K.; Yang, X.R.; Lin, X.Y.; Zhu, Y.G. Partial replacement of inorganic phosphorus (P) by organic manure reshapes phosphate mobilizing bacterial community and promotes P bioavailability in a paddy soil. Sci. Total Environ. 2020, 703, 134977. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Nicolardot, B.; Recous, S.; Mary, B. Simulation of C and N mineralisation during crop residue decomposition: A simple dynamic model based on the C:N ratio of the residues. Plant Soil 2001, 228, 83–103. [Google Scholar] [CrossRef]
- Tang, X.; Bernard, L.; Brauman, A.; Daufresne, T.; Deleporte, P.; Desclaux, D.; Souche, G.; Placella, S.A.; Hinsinger, P. Increase in microbial biomass and phosphorus availability in the rhizosphere of intercropped cereal and legumes under field conditions. Soil Biol. Biochem. 2014, 75, 86–93. [Google Scholar] [CrossRef]
- Chen, J.; Seven, J.; Zilla, T.; Dippold, M.A.; Blagodatskaya, E.; Kuzyakov, Y. Microbial C: N: P stoichiometry and turnover depend on nutrients availability in soil: A 14C, 15N and 33P triple labelling study. Soil Biol. Biochem. 2019, 131, 206–216. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol. Biochem. 2013, 61, 69–75. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Global Change Biol. 2014. 20, 1366–1381. [CrossRef]
- Chen, X.; Hao, B.; Jing, X.; He, J.S.; Ma, W.; Zhu, B. Minor responses of soil microbial biomass, community structure and enzyme activities to nitrogen and phosphorus addition in three grassland ecosystems. Plant Soil 2019, 444, 21–37. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Wang, L.; Jin, S.; Zhu, W.; Lu, Y.; Wang, S. A five-year P fertilization pot trial for wheat only in a rice-wheat rotation of Chinese paddy soil: Interaction of P availability and microorganism. Plant Soil 2016, 399, 305–318. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Wang, X.; Xu, M.; Wang, X.; Wei, X.; Zhu, Z.K.; Ge, T.D.; Peng, S.S.; Zhu, B.; et al. Decreasing microbial phosphorus limitation increases soil carbon release. Geoderma 2022, 419, 115868. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef]
- Wen, Z.; White, P.J.; Shen, J.; Lambers, H. Linking root exudation to belowground economic traits for resource acquisition. New Phytol. 2022, 233, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, Z.; Hu, S.; Chen, G.; Huang, R.; Wu, Y.; Li, B.; Tao, Q.; Jin, K.; Wang, C.; et al. Crop domestication disrupts intercropping benefits: A case study from barley-faba bean mixture under contrasting P inputs. Front. Plant Sci. 2023, 14, 1153237. [Google Scholar] [CrossRef]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Zhao, Z.; Xu, S.; Cai, S.; Zhu, H.; Rengel, Z.; Kuzyakov, Y. Carbon-Phosphorus coupling governs microbial effects on nutrient acquisition strategies by four crops. Front. Plant Sci. 2022, 13, 924154. [Google Scholar] [CrossRef]
- Wang, K.; Ren, T.; Yan, J.; Zhu, D.; Liao, S.; Zhang, Y.; Lu, Z.; Cong, R.; Li, X.; Lu, J. Straw returning mediates soil microbial biomass carbon and phosphorus turnover to enhance soil phosphorus availability in a rice-oilseed rape rotation with different soil phosphorus levels. Agr. Ecosyst. Environ. 2022, 335, 107991. [Google Scholar] [CrossRef]
- Damon, P.M.; Bowden, B.; Rose, T.; Rengel, Z. Crop residue contributions to phosphorus pools in agricultural soils: A review. Soil Biol. Biochem. 2024, 74, 127–137. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, J.; Li, P.; Tang, Y.; Lu, Y.; Liao, Y.; Nie, J. Enhancing phosphorus transformation in typical reddish paddy soil from China: Insights on long-term straw return and pig manure application via microbial mechanisms. Sci. Total Environ. 2024, 940, 173513. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Keller, B.; Hoop, D.; Jud, K.; Boivin, P.; Frossard, E. Increased availability of phosphorus after drying and rewetting of a grassland soil: Processes and plant use. Plant Soil 2013, 370, 511–526. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus forms and P-sorption properties in three alkaline soils after long-term mineral and manure applications in north-eastern Italy. Agric. Ecosyst. Environ. 2011, 141, 58–66. [Google Scholar] [CrossRef]
- He, Z.; Yang, X.; Xiang, J.; Wu, Z.; Shi, X.; Gui, Y.; Liu, M.; Kalkhajeh, Y.K.; Gao, H.; Ma, C. Does straw returning amended with straw decomposing microorganism inoculants increase the soil major nutrients in China’s farmlands? Agronomy 2022, 12, 890. [Google Scholar] [CrossRef]
- Jing, Z.; Chen, R.; Wei, S.; Feng, Y.; Zhang, J.; Lin, X. Response and feedback of C mineralization to P availability driven by soil microorganisms. Soil Biol. Biochem. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Y.; Zhou, J.; Yu, D.; Sun, H.; Wu, Y.; Zhou, J.; Yu, D.; Chen, Y. Microorganisms drive stabilization and accumulation of organic phosphorus: An incubation experiment. Soil Biol. Biochem. 2022, 172, 108750. [Google Scholar] [CrossRef]
- Peng, Y.; Duan, Y.; Huo, W.; Zhang, Z.; Huang, D.; Xu, M.; Feng, G. C: P stoichiometric imbalance between soil and microorganisms drives microbial phosphorus turnover in the rhizosphere. Biol. Fertil. Soils 2022, 58, 421–433. [Google Scholar] [CrossRef]
- Tang, X.; Placella, S.A.; Daydé, F.; Bernard, L.; Robin, A.; Journet, E.P.; Justes, E.; Hinsinger, P. Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertilizer gradient. Plant Soil 2016, 407, 119–134. [Google Scholar] [CrossRef]
- Zhang, D.; Kuzyakov, Y.; Zhu, H.; Alharbi, H.A.; Li, H.; Rengel, Z. Increased microbial biomass and turnover underpin efficient phosphorus acquisition by Brassica chinensis. Soil Tillage Res. 2022, 223, 105492. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Li, H.; Zhang, Y.; Xu, S.; Cai, S.; Sulaiman, A.A.; Kuzyakov, Y.; Rengel, Z.; Zhang, D. Dynamics of root–microbe interactions governing crop phosphorus acquisition after straw amendment. Soil Biol. Biochem. 2023, 181, 109039. [Google Scholar] [CrossRef]
- Qiu, S.; Mccomb, A.J.; Bell, R.W. Ratios of C, N and P in soil water direct microbial immobilization-mineralisation and N availability in nutrient amended sandy soils in southwestern Australia. Agric. Ecosyst. Environ. 2008, 127, 93–99. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Hu, Y.; Zhou, P.; Wang, T.; Shibistova, O.; Guggenberger, G.; Su, Y.; Wu, J. Fate of rice shoot and root residues, rhizodeposits, and microbial assimilated carbon in paddy soil-part 2: Turnover and microbial utilization. Plant Soil 2017, 416, 243–257. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2013. [Google Scholar]
- Ohno, T.; Zibilske, L.M. Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci. Soc. Am. J. 1991, 55, 892–895. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; The American Society of Agronomy, Inc.: Madison, WI, USA, 1982; Volume 9, pp. 643–698. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Vance, E.; Brookes, P.; Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Mori, T.; Aoyagi, R.; Kitayama, K.; Mo, J. Does the ratio of β-1,4-glucosidase to β-1,4-N-acetylglucosaminidase indicate the relative resource allocation of soil microbes to C and N acquisition? Soil Biol. Biochem. 2021, 160, 108363. [Google Scholar] [CrossRef]
- Neumann, G. Quantitative determination of acid phosphatase activity in the rhizosphere and on the root surface. In Handbook of Methods Used in Rhizosphere Research; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland, 2006; pp. 79–85. [Google Scholar]
- McGill, W.B.; Cannon, K.R.; Robertson, J.A.; Cook, D. Dynamics of soil microbial biomass and water-soluble organic C in Breton L after 50 years of cropping to two rotations. Can. J. Soil Sci. 1986, 66, 1–19. [Google Scholar] [CrossRef]


| Treatment | Phosphorus Activation Coefficient (PAC 1, %) | |||
|---|---|---|---|---|
| 0–10 cm | 10–20 cm | 20–30 cm | 30–40 cm | |
| CK | 20.4 ± 0.5 b | 19.3 ± 0.2 ab | 5.16 ± 0.64 b | 2.16 ± 0.32 a |
| NPK | 22.6 ± 0.4 b | 22.3 ± 0.7 a | 4.14 ± 0.51 b | 2.14 ± 0.55a |
| 0.75NPK | 22.2 ± 0.6 b | 16.8 ± 0.3 b | 1.75 ± 0.57 b | 1.67 ± 0.28 a |
| SNPK | 68.3 ± 0.1 a | 21.8 ± 0.1 a | 11.7 ± 0.6 a | 1.56 ± 0.17 a |
| Treatment | Average MBP (mg kg−1) | Total Released P (mg kg−1) | Turnover Rate (Year−1) | Annual MBP Flux (kg P ha−1 year−1) |
|---|---|---|---|---|
| CK | 10.3 ± 0.5 | 4.8 ± 2.6 | 0.58 ± 0.23 | 12.0 ± 3.4 |
| NPK | 11.3 ± 0.6 | 5.9 ± 1.0 | 0.52 ± 0.14 | 14.7 ± 1.8 |
| 0.75NPK | 12.5 ± 1.0 | 6.3 ± 1.1 | 0.53 ± 0.09 | 16.3 ± 3.8 |
| SNPK | 17.6 ± 0.6 | 8.9 ± 1.5 | 0.60 ± 0.1 | 21.3 ± 4.5 |
| Significance level (p) | ||||
| Fertilizer | 0.002 | 0.015 | 0.578 | 0.013 |
| Treatments | pH | SOC (g kg−1) | TN (g kg−1) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|
| CK | 5.53 (0.18) | 1.30 (0.04) | 1.0 (0.11) | 99.5 (9.2) | 10.5 (1.3) | 71.5 (10.2) |
| NPK | 5.54 (0.20) | 1.32 (0.02) | 1.5 (0.21) | 119.8 (13.2) | 12.6 (1.5) | 73.9 (11.5) |
| 0.75NPK | 5.62 (0.04) | 1.33 (0.04) | 1.3 (0.13) | 102.3 (11.2) | 13.2 (2.2) | 75.1 (8.9) |
| SNPK | 6.02 (0.05) | 1.52 (0.02) | 1.2 (0.21) | 125.2 (33.2) | 15.6 (2.1) | 79.3 (6.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zhou, Y.; Wu, R.; Wu, K.; Zhao, H.; Wang, W.; Zhang, Y.; Huang, R.; Wu, Y.; Li, B.; et al. Long-Term Straw Returning Enhances Phosphorus Uptake by Zea mays L. through Mediating Microbial Biomass Phosphorus Turnover and Root Functional Traits. Plants 2024, 13, 2389. https://doi.org/10.3390/plants13172389
Tang X, Zhou Y, Wu R, Wu K, Zhao H, Wang W, Zhang Y, Huang R, Wu Y, Li B, et al. Long-Term Straw Returning Enhances Phosphorus Uptake by Zea mays L. through Mediating Microbial Biomass Phosphorus Turnover and Root Functional Traits. Plants. 2024; 13(17):2389. https://doi.org/10.3390/plants13172389
Chicago/Turabian StyleTang, Xiaoyan, Yuxin Zhou, Runjuan Wu, Kuilin Wu, Hui Zhao, Wanyi Wang, Yanyan Zhang, Rong Huang, Yingjie Wu, Bing Li, and et al. 2024. "Long-Term Straw Returning Enhances Phosphorus Uptake by Zea mays L. through Mediating Microbial Biomass Phosphorus Turnover and Root Functional Traits" Plants 13, no. 17: 2389. https://doi.org/10.3390/plants13172389




