Genome-Wide Identification and Analysis of Maize DnaJ Family Genes in Response to Salt, Heat, and Cold at the Seedling Stage
Abstract
:1. Introduction
2. Results
2.1. ZmDnaJ Gene Family in the Maize Genome and Their Physiochemical Properties
2.2. Phylogenetic and Conserved Domain Analysis of the ZmDnaJ Gene Family
2.3. Conserved Motif and Gene Structure Analysis of the ZmDnaJ Gene Family
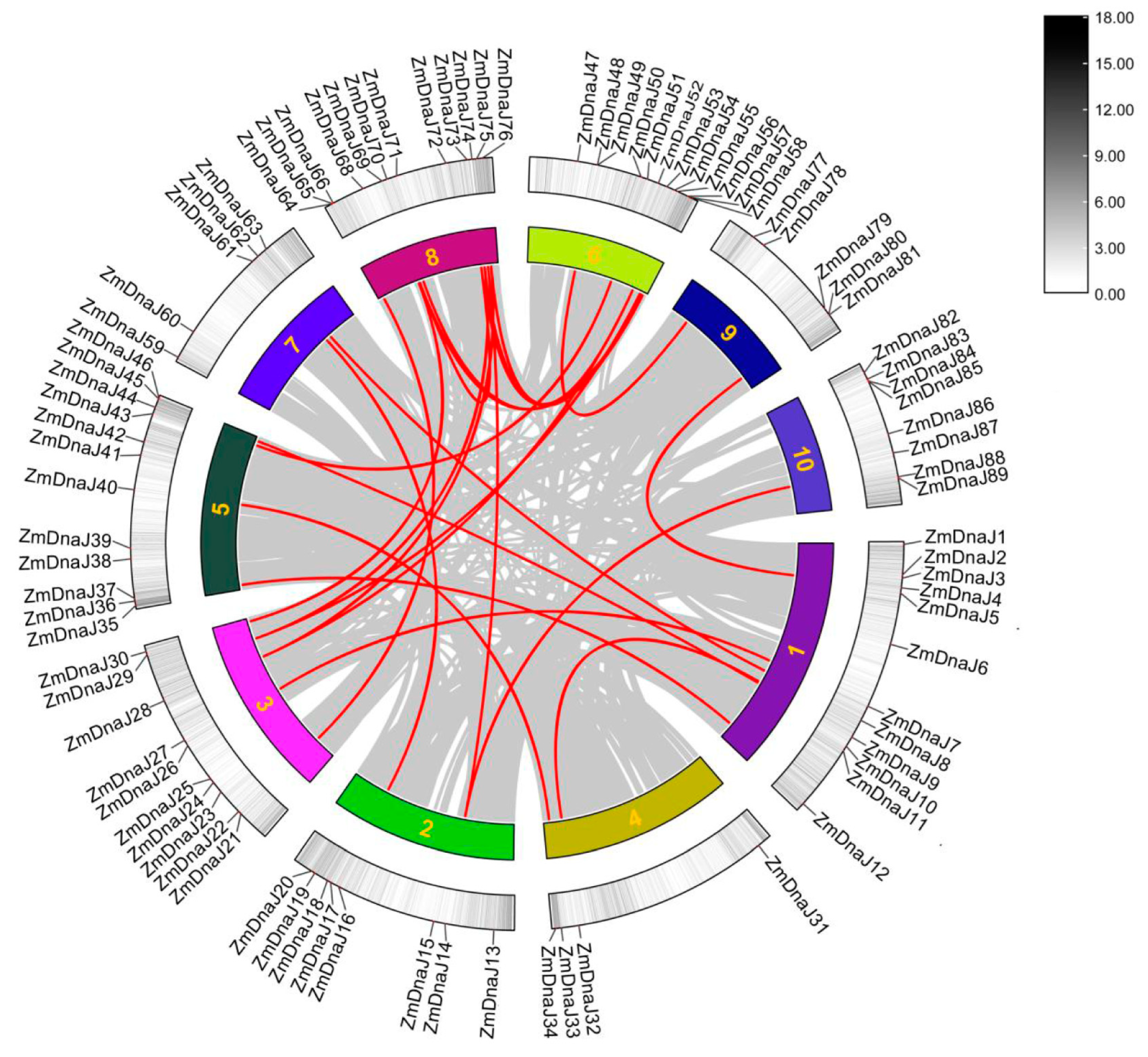
2.4. Gene Duplication Analysis of the ZmDnaJ Gene Family
2.5. Cis-Regulating Elements in Maize ZmDnaJ Promoters
2.6. Expression Patterns of ZmDnaJ Gene Family Members in Different Tissues
2.7. Expression Patterns of ZmDnaJ Gene Family Members under Abiotic Stress
2.8. Expression Patterns of 17 ZmDnaJs of Five Inbred Maize Lines under Heat Stress
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification of ZmDnaJ Gene Family in Maize
4.2. Phylogenetic Tree and Conserved Domain Analysis of ZmDnaJ Gene Family
4.3. Analysis of Protein Motif, Gene Structure, and Gene Duplication of the ZmDnaJ Gene Family
4.4. Cis-Acting Element Analysis of ZmDnaJ Genes
4.5. Expression of ZmDnaJ Genes Based on RNA-Seq in Maize cv. B73
4.6. Plant Material and Stress Treatment
4.7. RNA Extraction, and Quantitative Real-Time PCR Analysis (qRT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godara, H.; Ramakrishna, W. Endophytes as nature’s gift to plants to combat abiotic stresses. Lett. Appl. Microbiol. 2023, 76, ovac067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Piao, S.L.; Wang, X.H.; Lobell, D.B.; Huang, Y.; Huang, M.T.; Yao, Y.T.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.U.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, M.T.; Wang, Y.; Zhou, Z.S.; Wan, F.H.; Guo, J.Y. Induced thermotolerance and expression of three key Hsp genes (Hsp70, Hsp21, and sHsp21) and their roles in the high temperature tolerance of Agasicles hygrophila. Front. Physiol. 2020, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Walsh, P.; Bursac, D.; Law, Y.C.; Cyr, D.; Lithgow, T. The J-protein family: Modulating protein assembly, disassembly and translocation. EMBO Rep. 2004, 5, 567–571. [Google Scholar] [CrossRef]
- Rajan, V.B.; D’Silva, P. Arabidopsis thaliana J-class heat shock proteins: Cellular stress sensors. Funct. Integr. Genom. 2009, 9, 433–446. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Thapar, U.; Kundnani, P.; Panwar, P.; Grover, A. Functional relevance of J-protein family of rice (Oryza sativa). Cell Stress Chaperones 2013, 18, 321–331. [Google Scholar] [CrossRef]
- Craig, E.A.; Huang, P.; Aron, R.; Andrew, A. The diverse roles of J-proteins, the obligate Hsp70 co-chaperone. Rev. Physiol. Biochem. Pharmacol. 2006, 156, 1–21. [Google Scholar]
- Verma, A.K.; Diwan, D.; Raut, S.; Dobriyal, N.; Brown, R.E.; Gowda, V.; Hines, J.K.; Sahi, C. Evolutionary conservation and emerging functional diversity of the cytosolic Hsp70:J protein chaperone network of Arabidopsis thaliana. G3 2017, 7, 1941–1954. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Z.C.; Ouyang, M.; Xu, X.M.; Xiong, H.B.; Wang, Q.; Grimm, B.; Rochaix, J.D.; Zhang, L.X. The DnaJ proteins DJA6 and DJA5 are essential for chloroplast iron-sulfur cluster biogenesis. EMBO J. 2021, 40, e106742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jia, T.; Jiao, Q.S.; Hu, X.Y. Research Progress in J-Proteins in the Chloroplast. Genes 2022, 13, 1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.M.; Holmström, M.; Raksajit, W.; Suorsa, M.; Piippo, M.; Aro, E.M. Small chloroplast-targeted DnaJ proteins are involved in optimization of photosynthetic reactions in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Qin, Y.X.; Xie, C.G.; Zhao, F.Y.; Zhao, J.F.; Liu, D.F.; Chen, S.Y.; Fuglsang, A.T.; Palmgren, M.G.; Schumaker, K.S.; et al. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 2010, 22, 1313–1332. [Google Scholar] [CrossRef]
- Wang, G.D.; Cai, G.H.; Xu, N.; Zhang, L.T.; Sun, X.L.; Guan, J.; Meng, Q.W. Novel DnaJ protein facilitates thermotolerance of transgenic tomatoes. Int. J. Mol. Sci. 2019, 20, 367. [Google Scholar] [CrossRef]
- Kong, F.Y.; Deng, Y.S.; Wang, G.D.; Wang, J.R.; Liang, X.Q.; Meng, Q.W. LeCDJ1, a chloroplast DnaJ protein, facilitates heat tolerance in transgenic tomatoes. J. Integr. Plant Biol. 2014, 56, 63–74. [Google Scholar] [CrossRef]
- Kong, F.Y.; Deng, Y.S.; Zhou, B.; Wang, G.D.; Wang, Y.; Meng, Q.W. A chloroplast-targeted DnaJ protein contributes to maintenance of photosystem II under chilling stress. J. Exp. Bot. 2014, 65, 143–158. [Google Scholar] [CrossRef]
- Wang, G.D.; Cai, G.H.; Kong, F.Y.; Deng, Y.S.; Ma, N.N.; Meng, Q.W. Overexpression of tomato chloroplast-targeted DnaJ protein enhances tolerance to drought stress and resistance to Pseudomonas solanacearum in transgenic tobacco. Plant Physiol. Biochem. 2014, 82, 95–104. [Google Scholar] [CrossRef]
- Yamamoto, M.; Uji, S.; Sugiyama, T.; Sakamoto, T.; Kimura, S.; Endo, T.; Nishikawa, S.I. ERdj3B-Mediated Quality Control Maintains Anther Development at High Temperatures. Plant Physiol. 2020, 182, 1979–1990. [Google Scholar] [CrossRef]
- Leng, Y.J.; Yao, Y.S.; Yang, K.Z.; Wu, P.X.; Xia, Y.X.; Zuo, C.R.; Luo, J.H.; Wang, P.; Liu, Y.Y.; Zhang, X.Q.; et al. Arabidopsis ERdj3B coordinates with ERECTA-family receptor kinases to regulate ovule development and the heat stress response. Plant Cell 2022, 34, 3665–3684. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Z.; Xia, C.; Liu, X.L.; Dou, X.Y.; Wang, W.; Chen, L.Q.; Zhang, X.Q.; Xie, L.F.; He, L.; Ma, X.; et al. A mutation in Thermosensitive Male Sterile 1, encoding a heat shock protein with DnaJ and PDI domains, leads to thermosensitive gametophytic male sterility in Arabidopsis. Plant J. 2009, 57, 870–882. [Google Scholar] [CrossRef]
- Fan, F.F.; Yang, X.; Cheng, Y.; Kang, Y.Y.; Chai, X.R. The DnaJ Gene Family in Pepper (Capsicum annuum L.): Comprehensive Identification, Characterization and Expression Profiles. Front. Plant Sci. 2017, 8, 689. [Google Scholar] [CrossRef]
- Song, M.F.; Wang, X.; Zhang, K.J.; Chen, F.J.; Lou, Q.F. Identification of DnaJ gene family in cucumber and its expression response to high-temperature stress. J. Nanjing Agric. Univ. 2021, 44, 267–277. [Google Scholar]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Walley, J.W.; Sartor, R.C.; Shen, Z.; Schmitz, R.J.; Wu, K.J.; Urich, M.A.; Nery, J.R.; Smith, L.G.; Schnable, J.C.; Ecker, J.R.; et al. Integration of omic networks in a developmental atlas of maize. Science 2016, 353, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Makarevitch, I.; Waters, A.J.; West, P.T.; Stitzer, M.; Hirsch, C.N.; Ross-Ibarra, J.; Springer, N.M. Transposable elements contribute to activation of maize genes in response to abiotic stress. PLoS Genet. 2015, 11, e1004915. [Google Scholar]
- Xia, Z.L.; Zhang, X.Q.; Li, J.Q.; Su, X.H.; Liu, J.J. Overexpression of a tobacco J-domain protein enhances drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2014, 83, 100–106. [Google Scholar] [CrossRef]
- Wang, G.D.; Kong, F.Y.; Zhang, S.; Meng, X.; Wang, Y.; Meng, Q.W. A tomato chloroplast-targeted DnaJ protein protects Rubisco activity under heat stress. J. Exp. Bot. 2015, 66, 3027–3040. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhao, L.J.; Wang, J.H.; Lu, N.; Ma, W.J.; Ma, J.; Zhang, Y.; Fu, P.Y.; Yao, C.C.; Hu, J.W.; et al. Genome-wide identification of DnaJ gene family in Catalpa bungei and functional analysis of CbuDnaJ49 in leaf color formation. Front. Plant Sci. 2023, 14, 1116063. [Google Scholar]
- Nagaraju, M.; Kumar, A.; Rajasheker, G.; ManoharRao, D.; KaviKishor, P.B. DnaJs, the critical drivers of Hsp70s: Genome-wide screening, characterization and expression of DnaJ family genes in Sorghum bicolor. Mol. Biol. Rep. 2020, 47, 7379–7390. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.H.; Xu, Y.J.; Zhang, S.X.; Chen, T.T.; Liu, G.; Li, Z.Y.; Zhu, Y.S.; Wang, G.D. A tomato chloroplast-targeted DnaJ protein, SlDnaJ20 maintains the stability of photosystem I/II under chilling stress. Plant Signal. Behav. 2022, 17, 2139116. [Google Scholar] [CrossRef] [PubMed]
- Bekh-Ochir, D.; Shimada, S.; Yamagami, A.; Kanda, S.; Ogawa, K.; Nakazawa, M.; Matsui, M.; Sakuta, M.; Osada, H.; Asami, T.; et al. A novel mitochondrial DnaJ/Hsp40 family protein BIL2 promotes plant growth and resistance against environmental stress in brassinosteroid signaling. Planta 2013, 237, 1509–1525. [Google Scholar] [CrossRef]
- Cheetham, M.E.; Caplan, A.J. Structure, function and evolution of DnaJ: Conservation and adaptation of chaperone function. Cell Stress Chaperones 1998, 3, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef]
- Navarro, A.; Barton, N.H. Chromosomal speciation and molecular divergence-accelerated evolution in rearranged chromosomes. Science 2003, 300, 321–324. [Google Scholar] [CrossRef]
- Varela, J.C.; Praekelt, U.M.; Meacock, P.A.; Planta, R.J.; Mager, W.H. The Saccharomyces cerevisiae HSP12 gene is activated by the high-osmolarity glycerol pathway and negatively regulated by protein kinase A. Mol. Cell. Biol. 1995, 15, 6232–6245. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Z.Q.; Li, F.F.; Li, X.X.; Ji, H.K.; Yang, S.S. The specific MYB binding sites bound by TaMYB in the GAPCp2/3 promoters are involved in the drought stress response in wheat. BMC Plant Biol. 2019, 19, 366. [Google Scholar] [CrossRef]
- Huang, B.R.; Xu, C.P. Identification and characterization of proteins associated with plant tolerance to heat stress. J. Integr. Plant Biol. 2008, 50, 1230–1237. [Google Scholar] [CrossRef]
- Hu, W.H.; Hu, G.C.; Han, B. Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci. 2009, 176, 583–590. [Google Scholar] [CrossRef]
- Fragkostefanakis, S.; Röth, S.; Schleiff, E.; Scharf, K.D. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ. 2015, 38, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom. 2007, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (ITOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Rui, X. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zeng, R.; Li, Z.Y.; Shi, Y.T.; Fu, D.Y.; Yin, P.; Cheng, J.K.; Jiang, C.F.; Yang, S.H. Natural variation in a type—A response regulator confers maize chilling tolerance. Nat Commun. 2021, 12, 4713. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





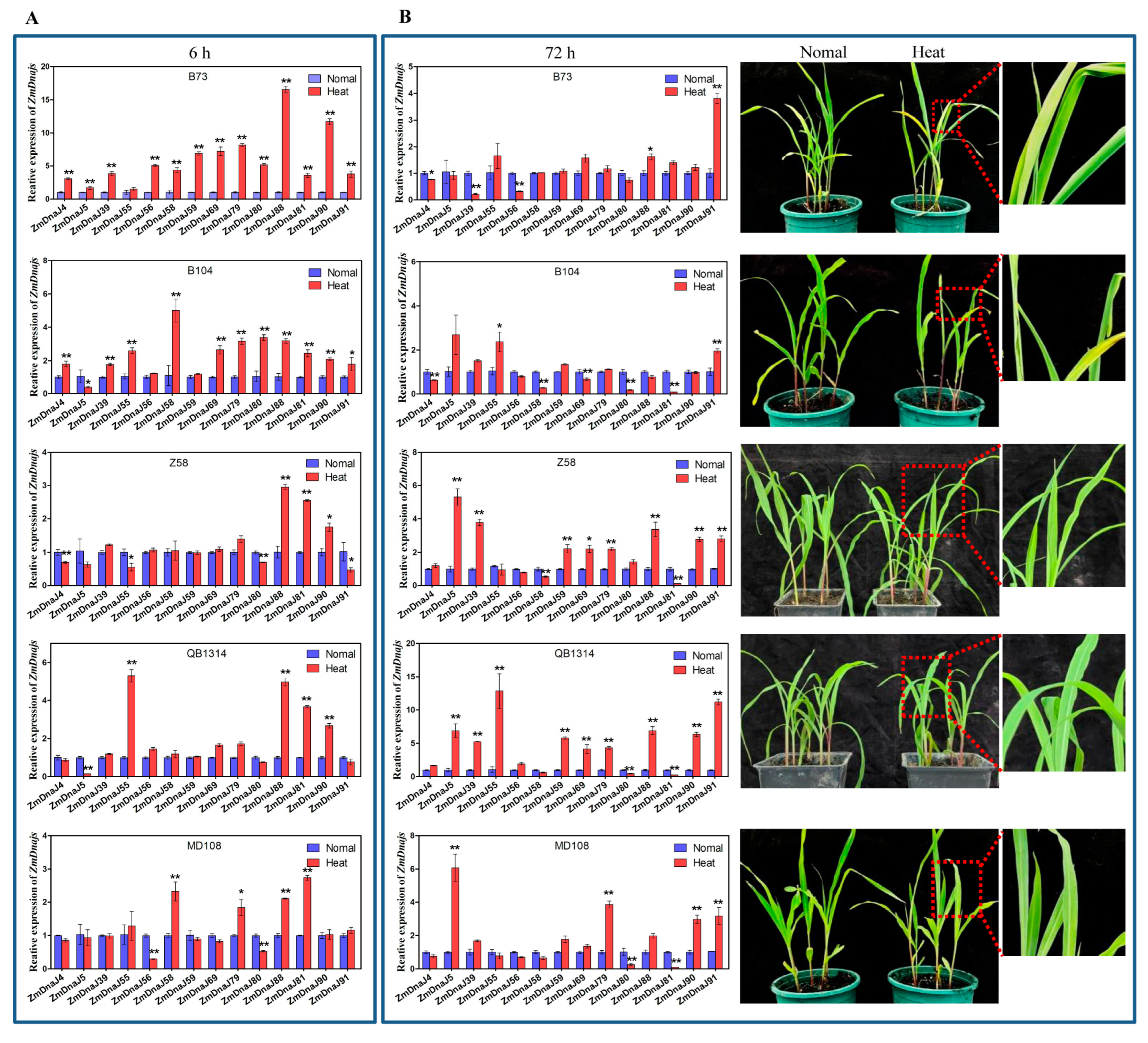
| Heat Stress Gene | Heat-Sensitive Inbred Lines | Heat-Tolerant Inbred Lines | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B73 | B104 | Z58 | QB1314 | MD108 | ||||||
| 6 h | 72 h | 6 h | 72 h | 6 h | 72 h | 6 h | 72 h | 6 h | 72 h | |
| ZmDnaJ4 | up | - | up | down | down | - | - | - | - | - |
| ZmDnaJ5 | up | - | down | - | - | up | down | up | - | down |
| ZmDnaJ39 | up | down | up | - | - | up | - | up | - | - |
| ZmDnaJ55 | up | - | up | up | down | up | up | - | - | |
| ZmDnaJ56 | up | down | - | - | - | - | - | down | - | |
| ZmDnaJ58 | up | - | up | down | - | down | - | up | - | |
| ZmDnaJ59 | up | - | - | - | - | up | - | up | - | up |
| ZmDnaJ69 | up | - | up | - | - | up | - | up | - | - |
| ZmDnaJ79 | up | - | up | - | - | up | - | up | up | up |
| ZmDnaJ80 | up | - | up | down | down | - | - | - | down | - |
| ZmDnaJ81 | up | - | up | down | up | down | up | down | up | - |
| ZmDnaJ88 | up | up | up | - | up | up | up | up | up | up |
| ZmDnaJ90 | up | - | up | - | up | up | up | up | - | up |
| ZmDnaJ91 | up | up | up | up | down | up | - | up | - | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Chen, Z.; Guo, X.; Tian, D.; Li, C.; Lin, M.; Hu, C.; Yan, J. Genome-Wide Identification and Analysis of Maize DnaJ Family Genes in Response to Salt, Heat, and Cold at the Seedling Stage. Plants 2024, 13, 2488. https://doi.org/10.3390/plants13172488
Li G, Chen Z, Guo X, Tian D, Li C, Lin M, Hu C, Yan J. Genome-Wide Identification and Analysis of Maize DnaJ Family Genes in Response to Salt, Heat, and Cold at the Seedling Stage. Plants. 2024; 13(17):2488. https://doi.org/10.3390/plants13172488
Chicago/Turabian StyleLi, Gang, Ziqiang Chen, Xinrui Guo, Dagang Tian, Chenchen Li, Min Lin, Changquan Hu, and Jingwan Yan. 2024. "Genome-Wide Identification and Analysis of Maize DnaJ Family Genes in Response to Salt, Heat, and Cold at the Seedling Stage" Plants 13, no. 17: 2488. https://doi.org/10.3390/plants13172488






