The F-Box Protein TaFBA1 Positively Regulates Drought Resistance and Yield Traits in Wheat
Abstract
:1. Introduction
2. Results
2.1. Generation and Identification of TaFBA1-Overexpressing and TaFBA1-RNAi Wheat Lines
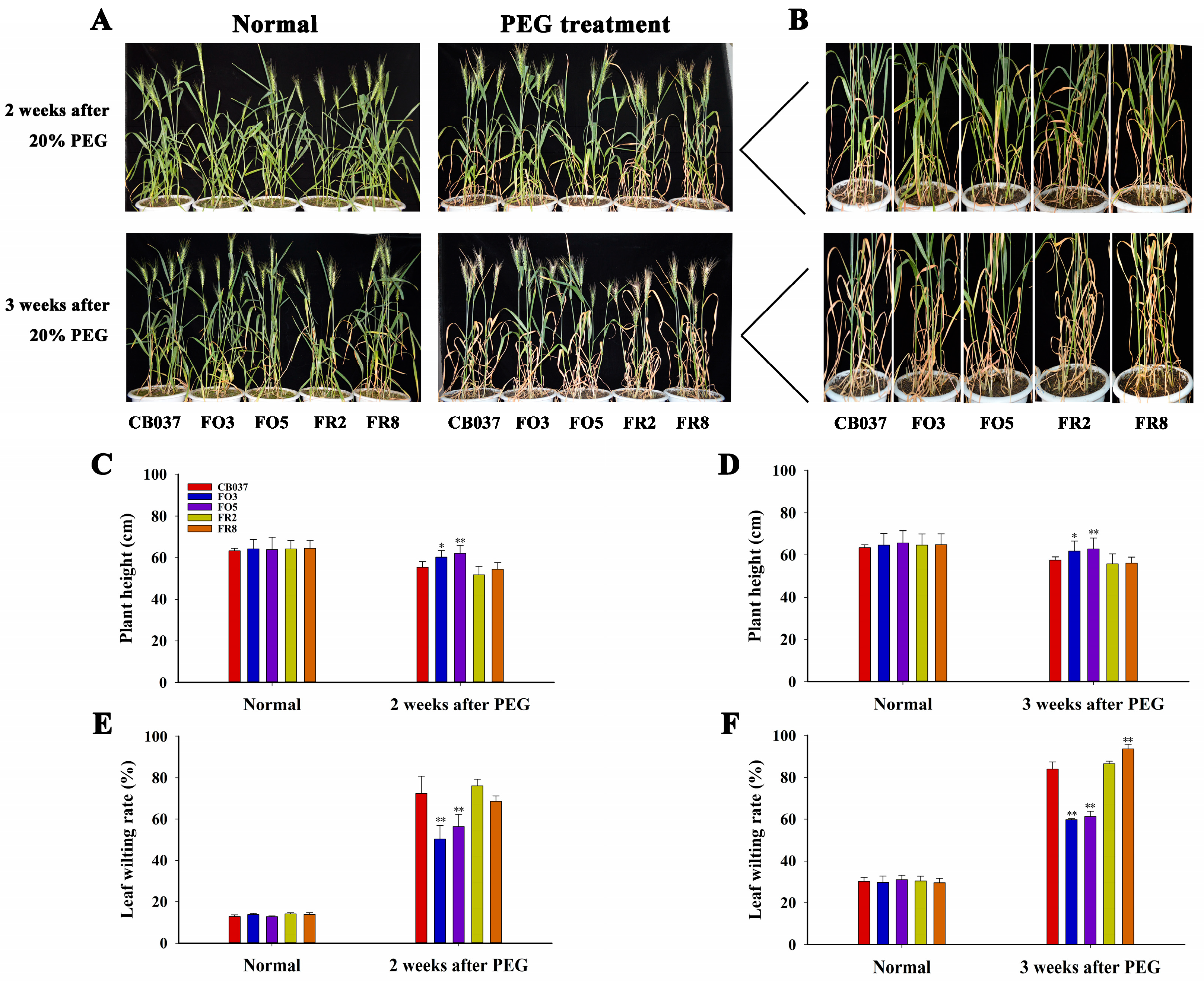
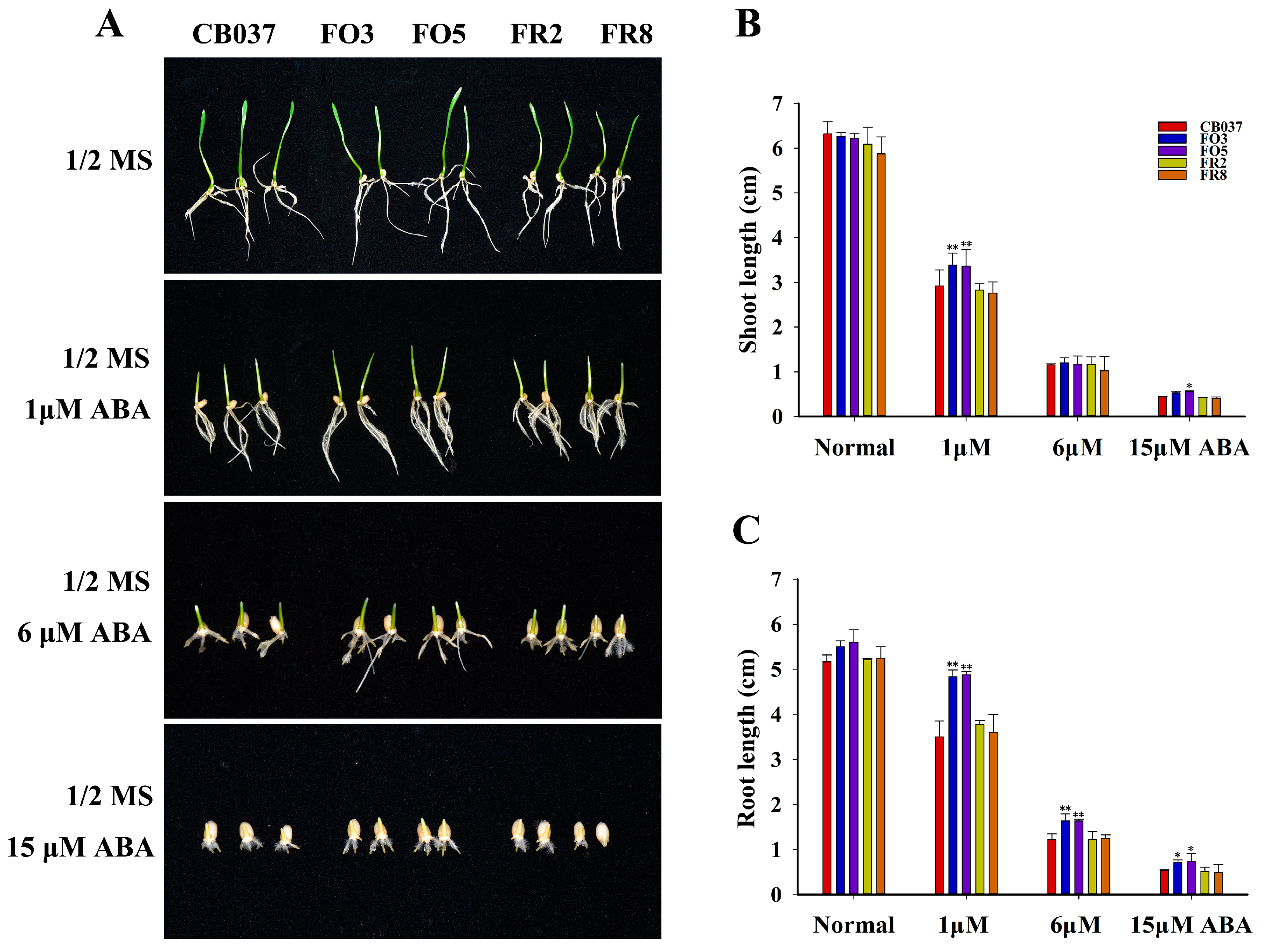
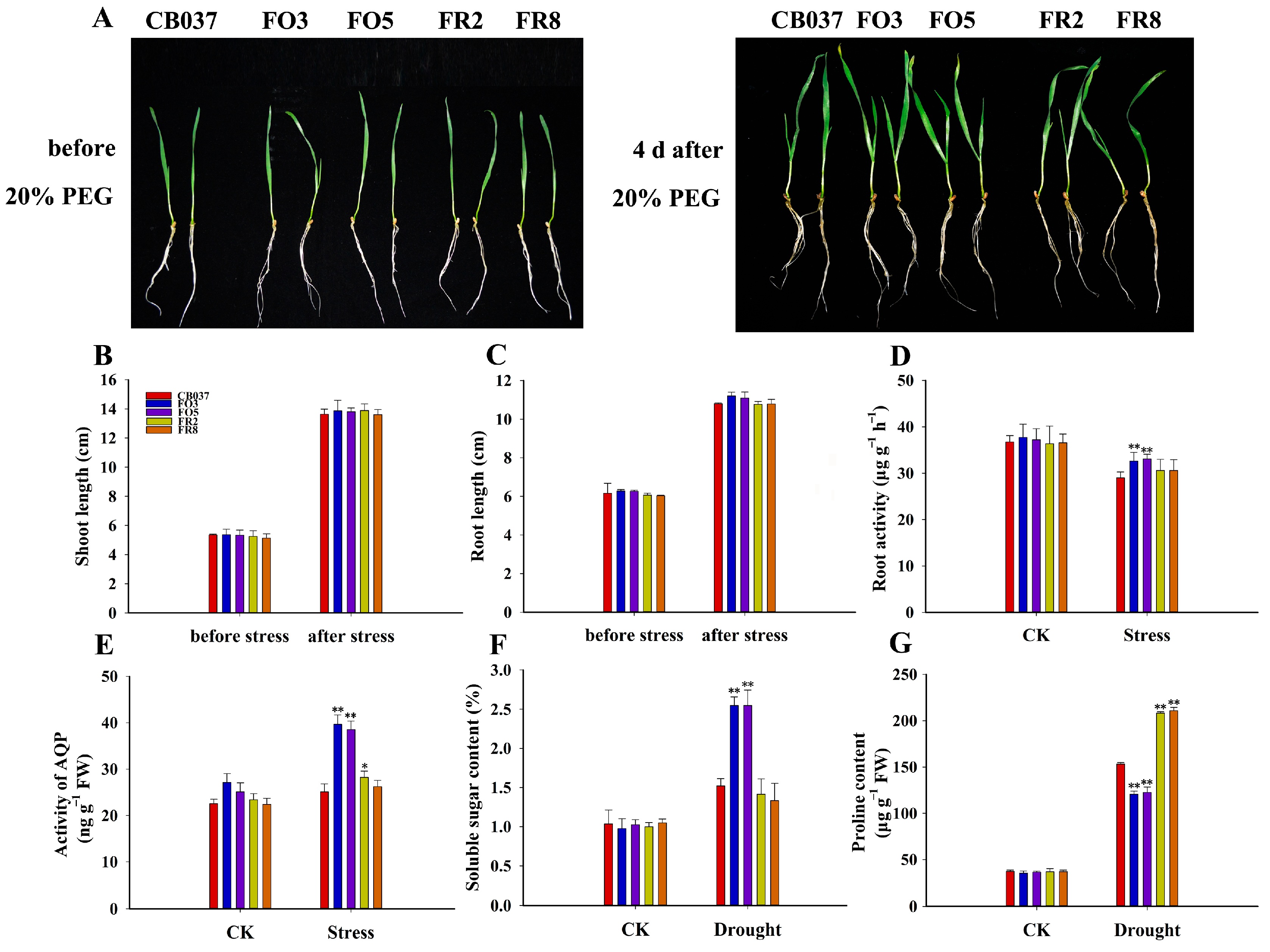
2.2. TaFBA1 Overexpression Confers Drought Tolerance to Wheat at Seedling and Heading Stages
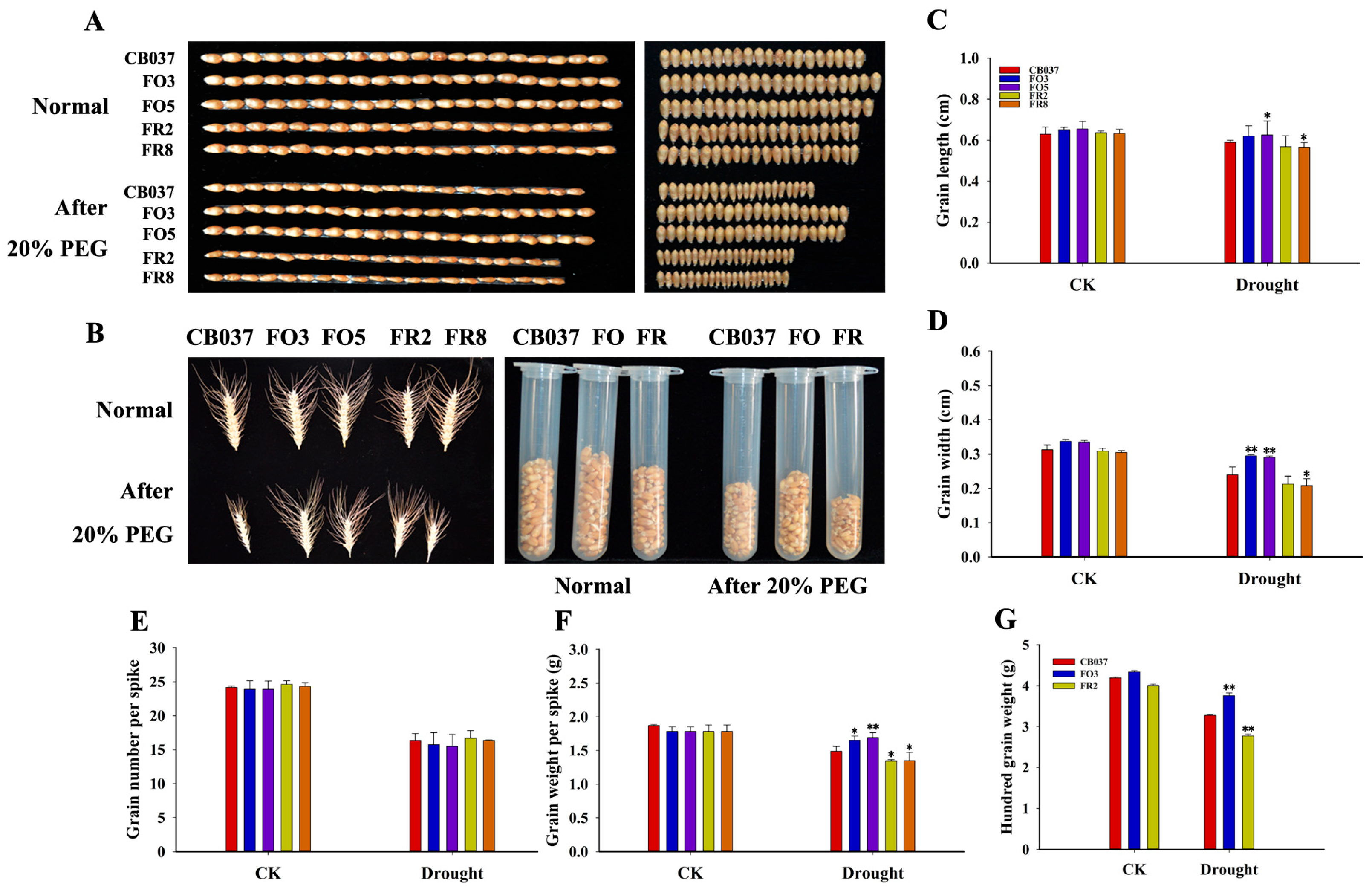
2.3. TaFBA1 Overexpression Impacted Spike Weight and Grain Size
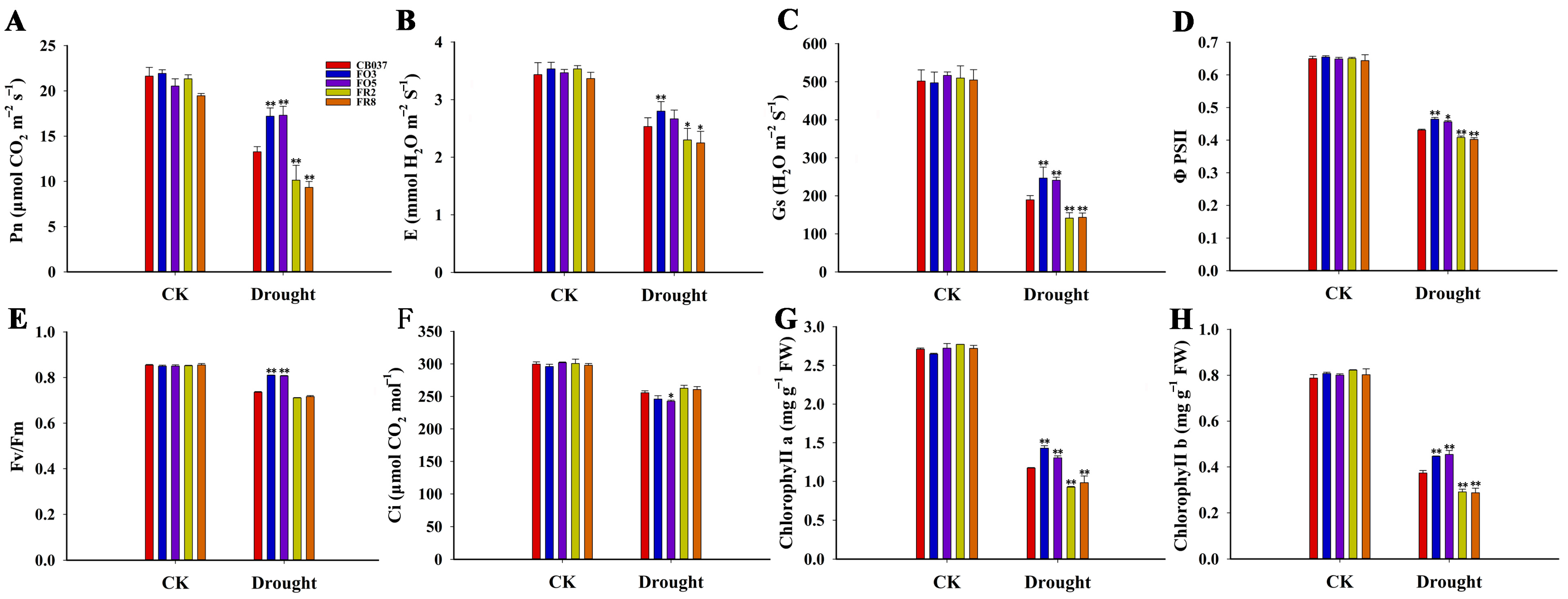
2.4. TaFBA1 Overexpression Improved the Photosynthetic Capacity of Transgenic Wheat under Drought Stress
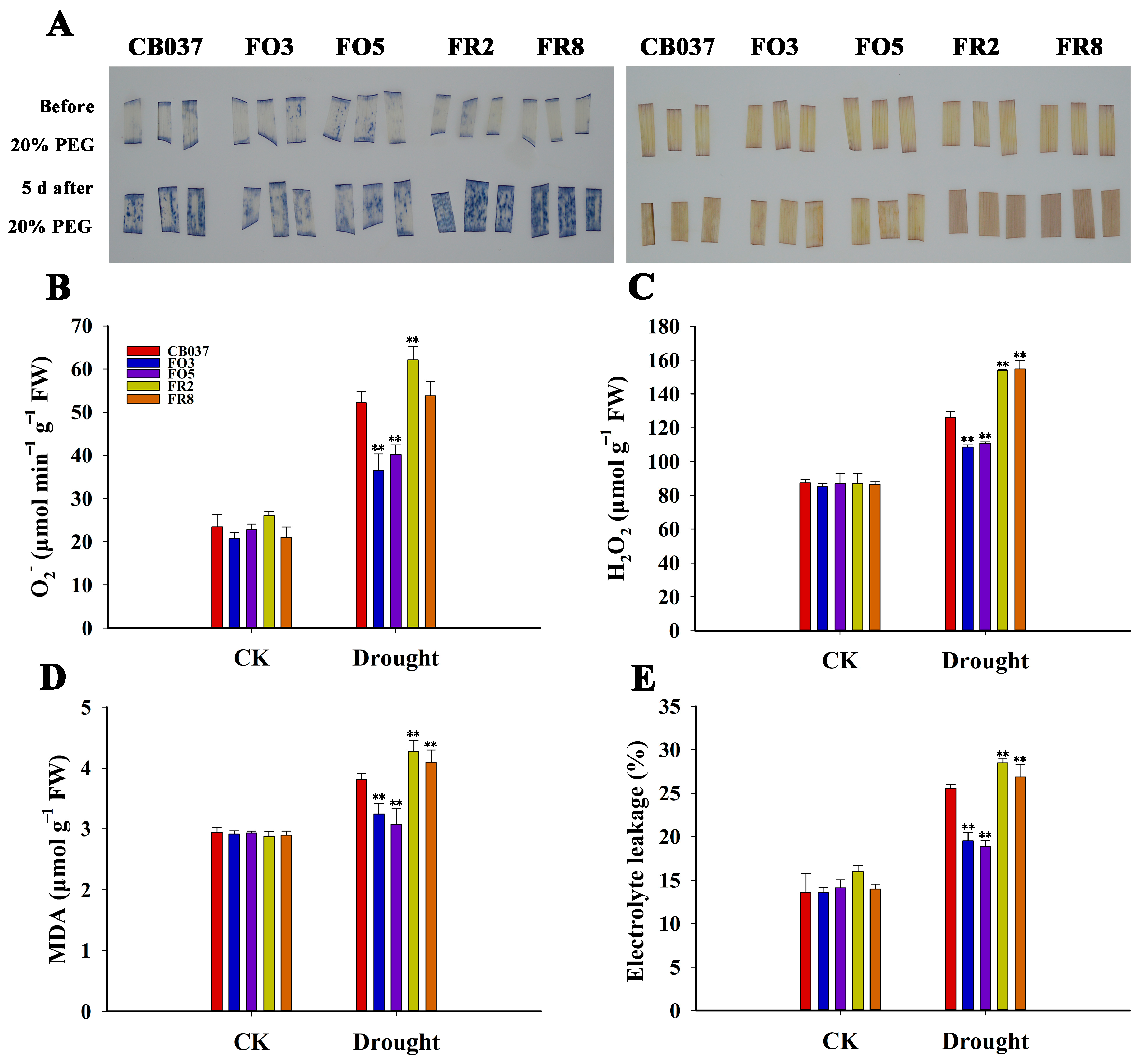
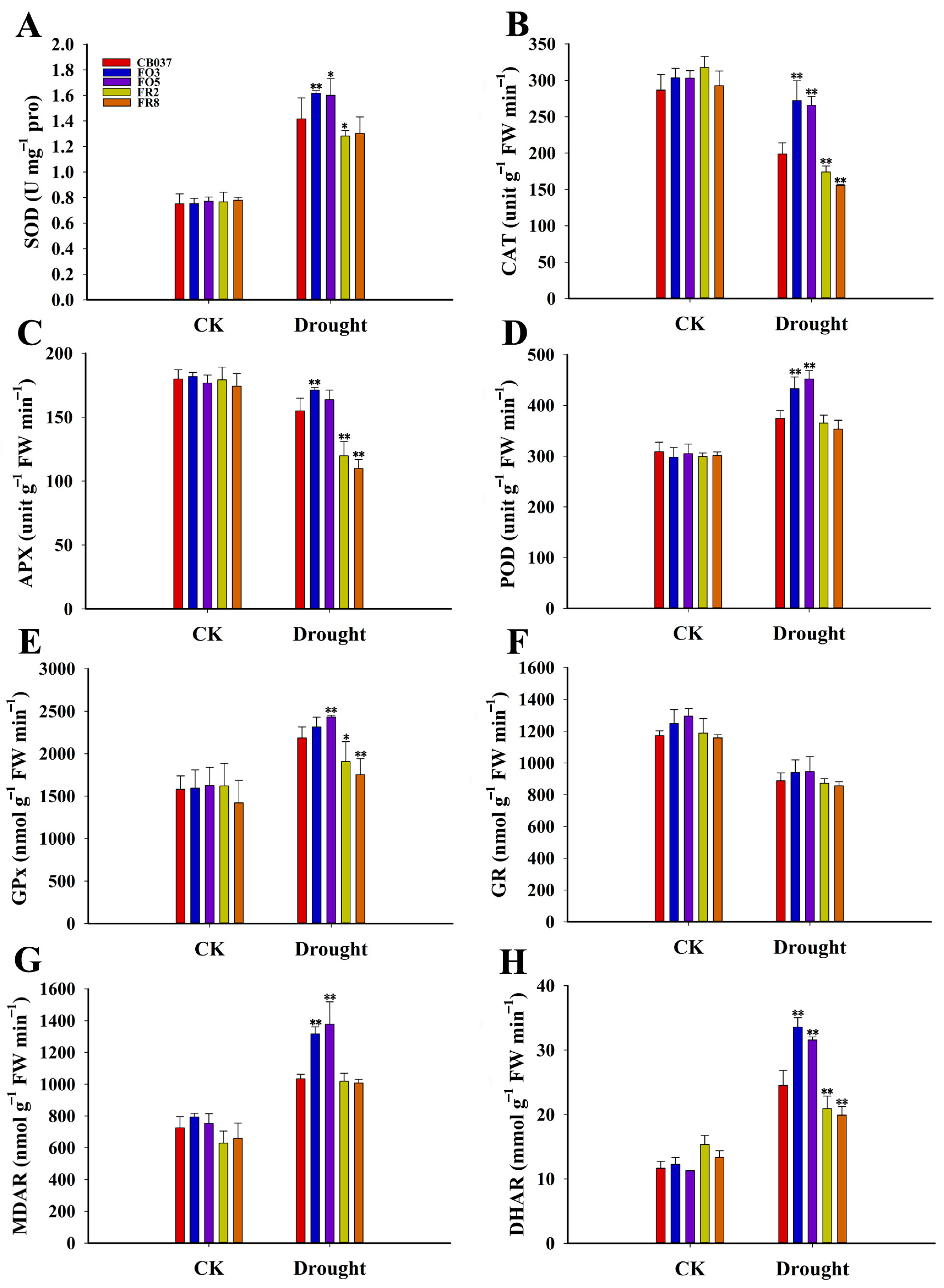
2.5. TaFBA1 Overexpression Alleviated Oxidative Damage in Wheat under Drought Stress
2.6. TaFBA1 Overexpression Enhanced the ROS Scavenging Capacity of Wheat under Drought Stress
2.7. TaFBA1 Overexpression Supported Stomatal Opening
2.8. TaFBA1 Overexpression Enhanced Root Water Absorption Capacity of Wheat under Drought Stress
2.9. Transcriptomic Analyses of CB037 and TaFBA1-OE Revealed Functions of TaFBA1 in Drought Tolerance
3. Discussion
3.1. TaFBA1 Positively Regulated the Drought Tolerance of Wheat
3.2. TaFBA1 Improved Drought Tolerance of Wheat by Enhanced Antioxidant Ability and Stress-Related Genes Regulation
3.3. Enhanced Root Water Absorption Capacity of TaFBA1-OE Wheat Made Contribution to Increased Drought Tolerance
3.4. TaFBA1 Increased Wheat Grain Size, Especially under Drought Stress
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Vector Construction, Generation of Transgenic Wheat and Verification of Transgenic Lines
4.3. Drought Stress Treatment
4.4. Chlorophyll Content and Photosynthetic Parameter Assays
4.5. Malondialdehyde Content, Electrolyte Leakage, Proline and Soluble Sugar Contents
4.6. Antioxidant Analysis Experiments
4.7. Stomatal Aperture
4.8. Seed Germination and Seedling Growth Assays
4.9. Water Loss Rate, Relative Water Content
4.10. RNA-Seq Assay
4.11. qRT-PCR Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langridge, P.; Reynolds, M. Breeding for drought and heat tolerance in wheat. Theor. Appl. Genet. 2021, 134, 1753–1769. [Google Scholar] [CrossRef]
- Ma, J.H.; Geng, Y.K.; Liu, H.; Zhang, M.Q.; Liu, S.J.; Hao, C.Y.; Hou, J.; Zhang, Y.F.; Zhang, D.J.; Zhang, W.J.; et al. TaTIP41 and TaTAP46 positively regulate drought tolerance in wheat by inhibiting PP2A activity. J. Integr. Plant Biol. 2023, 65, 2056–2070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zheng, Y.; Xiong, X.X.; Li, H.; Zhang, X.; Song, Y.L.; Zhang, X.H.; Min, D.H. The wheat VQ motif-containing protein TaVQ4-D positively regulates drought tolerance in transgenic plants. J. Exp. Bot. 2023, 74, 5591–5605. [Google Scholar] [CrossRef]
- Cui, L.G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice. PLoS Genet. 2015, 11, e1005617. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- An, J.; Li, Q.X.; Yang, J.J.; Zhang, G.Q.; Zhao, Z.X.; Wu, Y.Z.; Wang, Y.; Wang, W. Wheat F-box Protein TaFBA1 Positively Regulates Plant Drought Tolerance but Negatively Regulates Stomatal Closure. Front. Plant Sci. 2019, 10, 1242–1261. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Hu, W.; Zhou, Y.; Xiao, J.; Hu, R.; Wei, Q.H.; Zhang, Y.; Feng, J.L.; Sun, F.S.; Sun, J.T.; et al. TaASR1-D confers abiotic stress resistance by affecting ROS accumulation and ABA signalling in transgenic wheat. Plant Biotech. J. 2021, 19, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.D.; Jian, C.; Cheng, X.X.; Chen, B.; Mei, F.M.; Li, F.F.; Zhang, Y.F.; Li, S.M.; Du, L.Y.; Li, T.; et al. The wheat ABA receptor gene TaPYL1-1B contributes to drought tolerance and grain yield by increasing water-use efficiency. Plant Biotechnol. J. 2022, 20, 846–861. [Google Scholar] [CrossRef]
- Mei, F.M.; Chen, B.; Du, L.Y.; Li, S.M.; Zhu, D.H.; Chen, N.; Zhang, Y.F.; Li, F.F.; Wang, Z.X.; Cheng, X.X.; et al. A gain-of-function allele of a DREB transcription factor gene ameliorates drought tolerance in wheat. Plant Cell 2022, 34, 4472–4494. [Google Scholar] [CrossRef]
- Mao, H.D.; Li, S.M.; Chen, B.; Jian, C.; Mei, F.M.; Zhang, Y.F.; Li, F.F.; Chen, N.; Li, T.; Du, L.Y.; et al. Variation in cis-regulation of a NAC transcription factor contributes to drought tolerance in wheat. Mol. Plant 2022, 15, 276–292. [Google Scholar] [CrossRef]
- Finley, D.; Ulrich, H.D.; Sommer, T.; Kaiser, P. The ubiquitin–proteasome system of saccharomyces cerevisiae. Genetics 2012, 192, 319–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Wang, W.Q.; Wu, Y.Z.; Li, Q.X.; Zhang, G.Q.; Shi, R.R.; Yang, J.J.; Wang, Y.; Wang, W. The involvement of wheat U-box E3 ubiquitin ligase TaPUB1 in salt stress tolerance. J. Integr. Plant Biol. 2020, 62, 631–651. [Google Scholar] [CrossRef]
- Li, Q.X.; Wang, W.Q.; Wang, W.L.; Zhang, G.Q.; Liu, Y.; Wang, Y.; Wang, W. Wheat F-Box protein gene TaFBA1 is involved in plant tolerance to heat stress. Front. Plant Sci. 2018, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Cui, M.H.; Noh, M.S.; Jung, K.W.; Shin, J.S. The FBA motif-containing protein AFBA1 acts as a novel positive regulator of ABA response in Arabidopsis. Plant Cell Physiol. 2017, 58, 574–586. [Google Scholar] [CrossRef]
- Jia, F.J.; Wang, C.Y.; Huang, J.G.; Yang, G.D.; Wu, C.A.; Zheng, C.C. SCF E3 ligase PP2-B11 plays a positive role in response to salt stress in Arabidopsis. J. Exp. Bot. 2015, 66, 4683–4697. [Google Scholar] [CrossRef]
- Sharma, E.; Bhatnagar, A.; Bhaskar, A.; Majee, S.M.; Kieffer, M.; Kepinski, S.; Khurana, P.; Khurana, J.P. Stress-induced F-Box protein-coding gene OsFBX257 modulates drought stress adaptations and ABA responses in rice. Plant Cell Environ. 2023, 46, 1207–1231. [Google Scholar] [CrossRef]
- Li, Z.S.; Wang, X.Y.; Cao, X.C.; Chen, B.Z.; Ma, C.K.; Lv, J.Y.; Sun, Z.M.; Qiao, K.K.; Zhu, L.F.; Zhang, C.J.; et al. GhTULP34, a member of tubby-like proteins, interacts with GhSKP1A to negatively regulate plant osmotic stress. Genomics 2021, 113, 462–474. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Xu, W.; Li, Z.; Deng, X.W.; Wu, W.; Xue, Y. F-box protein DOR functions as a novel inhibitory factor for abscisic acid-induced stomatal closure under drought stress in Arabidopsis. Plant Physiol. 2008, 148, 2121–2133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.Y.; Luo, W.; Li, W.X.; Chen, N.; Zhang, D.J.; Chong, K. The F-Box Protein OsFBK12 Targets OsSAMS1 for Degradation and Affects Pleiotropic Phenotypes, Including Leaf Senescence, in Rice. Plant Physiol. 2013, 163, 1673–1685. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, X.; Yin, S. The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol. Biochem. 2014, 84, 213–223. [Google Scholar] [CrossRef]
- Al-Saharin, R.; Hellmann, H.; Mooney, S. Plant E3 Ligases and Their Role in Abiotic Stress Response. Cells 2022, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Li, B.W.; Gao, S.; Yang, Z.M.; Song, J.B. The F-box E3 ubiquitin ligase AtSDR is involved in salt and drought stress responses in Arabidopsis. Gene 2022, 809, 146011. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Z.; Zhou, S.M.; Yin, S.H.; Zhao, Z.X.; Han, Y.Y.; Wang, W. Stress-Inducible Expression of an F-box Gene TaFBA1 from Wheat Enhanced the Drought Tolerance in Transgenic Tobacco Plants without Impacting Growth and Development. Front. Plant Sci. 2016, 7, 1295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Zhang, G.Q.; Zhou, S.M.; Ren, Y.Q.; Wang, W. The improvement of salt tolerance in transgenic tobacco by overexpression of wheat F-box gene TaFBA1. Plant Sci. 2017, 259, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Xu, N.N.; Chu, Y.L.; Chen, H.L.; Li, X.X.; Wu, Q.; Jin, L.; Wang, G.X.; Huang, J.L. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA-mediated regulatory pathway and ROS scavenging. PLoS Genet. 2018, 14, e1007662. [Google Scholar] [CrossRef]
- Yu, H.; Chen, X.; Hong, Y.Y.; Wang, Y.; Xu, P.; Ke, S.D.; Liu, H.Y.; Zhu, J.K.; Oliver, D.J.; Xiang, C.B. Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. Plant Cell 2008, 20, 1134–1151. [Google Scholar] [CrossRef]
- Ma, H.Z.; Liu, C.; Li, Z.X.; Ran, Q.J.; Xie, G.N.; Wang, B.M.; Fang, S.; Chu, J.F.; Zhang, J.R. ZmbZIP4 contributes to stress resistance in maize by regulating ABA synthesis and root development. Plant Physiol. 2018, 178, 753–770. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhang, G.Q.; An, J.; Li, Q.X.; Chen, Y.H.; Zhao, X.Y.; Wu, J.J.; Wang, Y.; Hao, Q.Q.; Wang, W.Q.; et al. Expansin gene TaEXPA2 positively regulates drought tolerance in transgenic wheat (Triticum aestivum L.). Plant Sci. 2020, 298, 110596. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, B.; Qin, F. Arabidopsis RZFP34/CHYR1, a ubiquitin E3 ligase, regulates stomatal movement and drought tolerance via SnRK2.6-mediated phosphorylation. Plant Cell 2015, 27, 3228–3244. [Google Scholar] [CrossRef]
- Guo, C.K.; Yao, L.Y.; You, C.J.; Wang, S.S.; Cui, J.; Ge, X.C.; Ma, H. MID1 plays an important role in response to drought stress during reproductive development. Plant J. 2016, 88, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Kaouthar, F.; Ameny, F.K.; Yosra, K.; Walid, S.; Ali, G.; Faiçal, B. Responses of transgenic Arabidopsis plants and recombinant yeast cells expressing a novel durum wheat manganese superoxide dismutase TdMnSOD to various abiotic stresses. J. Plant Physiol. 2016, 198, 56–68. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Endler, A.; Kesten, C.; Schneider, R.; Zhang, Y.; Ivakov, A.; Froehlich, A.; Funke, N.; Persson, S. A mechanism for sustained cellulose synthesis during salt stress. Cell 2015, 162, 1353–1364. [Google Scholar] [CrossRef]
- Wang, T.; McFarlane, H.E.; Persson, S. The impact of abiotic factors on cellulose synthesis. J. Exp. Bot. 2016, 67, 543–552. [Google Scholar] [CrossRef]
- Lou, H.Y.; Tucker, M.R.; Shirley, N.J.; Lahnstein, J.; Yang, X.J.; Ma, C.; Schwerdt, J.; Fusi, R.; Burton, R.A.; Band, L.R.; et al. The cellulose synthase-like F3 (CslF3) gene mediates cell wall polysaccharide synthesis and affects root growth and differentiation in barley. Plant J. 2022, 110, 1681–1699. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.C.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.G.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct ω-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef]
- Fan, J.L.; Yu, L.H.; Xu, C.C. A central role for triacylglycerol in membrane lipid breakdown, fatty acid β-oxidation, and plant survival under extended darkness. Plant Physiol. 2017, 174, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Kesy, J.; Glazinska, P.; Glinkowski, W.; Narozna, D.; Bocianowski, J.; Rucinska-Sobkowiak, R.; Mai, V.C.; Krzesinski, W.; Samardakiewicz, S.; et al. The Influence of Lead and Acyrthosiphon pisum (Harris) on Generation of Pisum sativum Defense Signaling Molecules and Expression of Genes Involved in Their Biosynthesis. Int. J. Mol. Sci. 2023, 24, 10671. [Google Scholar] [CrossRef] [PubMed]
- López, M.A.; Vicente, J.; Kulasekaran, S.; Vellosillo, T.; Martínez, M.; Irigoyen, M.L.; Cascón, T.; Bannenberg, G.; Hamberg, M.; Castresana, C. Antagonistic role of 9-lipoxygenase-derived oxylipins and ethylene in the control of oxidative stress, lipid peroxidation and plant defence. Plant J. 2011, 67, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K.; Handa, A.K.; Mattoo, A.K. Transcript abundance patterns of 9-and 13-lipoxygenase subfamily gene members in response to abiotic stresses (heat, cold, drought or salt) in tomato (Solanum lycopersicum L.) highlights member-specific dynamics relevant to each stress. Genes 2019, 10, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, B.; Yang, C.L.; Wang, W.L.; Xiang, L.; Wang, Y.P.; Chan, Z.L. Jasmonic acid biosynthetic genes TgLOX4 and TgLOX5 are involved in daughter bulb development in tulip (Tulipa gesneriana). Hortic. Res. 2022, 11, 6–18. [Google Scholar] [CrossRef]
- Mega, R.; Abe, F.; Kim, J.S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J.; et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159. [Google Scholar] [CrossRef]
- Khandal, H.; Gupta, S.K.; Dwivedi, V.; Mandal, D.; Sharma, N.K.; Vishwakarma, N.K.; Pal, L.; Choudhary, M.; Francis, A.; Malakar, P.; et al. Root-specific expression of chickpea cytokinin oxidase/ dehydrogenase 6 leads to enhanced root growth, drought tolerance and yield without compromising nodulation. Plant Biotechnol. J. 2020, 18, 2225–2240. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, H.B.; Jia, X.M.; Li, X.W.; Mao, K.; Ma, F.W. The γ-clade HD-Zip I transcription factor MdHB-7 regulates salt tolerance in transgenic apple (Malus domestica). Plant Soil 2021, 463, 509–522. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2012, 63, 43–57. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, D.J.; Kim, Y.O.; Kim, J.S.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Uraji, M.; Watanabe-Sugimoto, M.; Okuma, E.; Munemasa, S.; Shimoishi, Y. FIA functions as an early signal component of abscisic acid signal cascade in Vicia faba guard cells. J. Exp. Bot. 2012, 63, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Saradadevi, R.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Stomatal behaviour under terminal drought affects post-anthesis water use in wheat. Funct. Plant Biol. 2017, 44, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, Y.J.; Liu, X.Y.; Tong, S.M.; Xing, J.W.; Zhang, Y.; Pudake, R.N.; Izquierdo, E.M.; Peng, H.R.; Xin, M.M.; et al. The E3 ligase TaSAP5 alters drought stress responses by promoting the degradation of DRIP proteins. Plant Physiol. 2017, 175, 1878–1892. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Lin, D.X.; Zhang, Y.W.; Deng, M.; Chen, Y.X.; Lv, B.; Li, B.S.; Lei, Y.; Wang, Y.P.; Zhao, L.; et al. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y. Signaling pathways of seed size control in plants. Curr. Opin. Plant Biol. 2016, 33, 23–32. [Google Scholar] [CrossRef]
- Miller, C.; Wells, R.; McKenzie, N.; Trick, M.; Ball, J.; Fatihi, A.; Dubreucq, B.; Chardot, T.; Lepiniec, L.; Bevan, M.W. Variation in expression of the HECT E3 ligase UPL3 modulates LEC2 levels, seed size, and crop yields in Brassica napus. Plant Cell 2019, 31, 2370–2385. [Google Scholar] [CrossRef]
- Liu, L.C.; Tong, H.G.; Xiao, Y.H.; Che, R.H.; Xu, F.; Hu, B.; Liang, C.; Chu, J.; Li, J.; Chu, C. Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11102–11107. [Google Scholar] [CrossRef]
- Brinton, J.; Simmonds, J.; Minter, F.; Leverington-Waite, M.; Snape, J.; Uauy, C. Increased pericarp cell length underlies a major quantitative trait locus for grain weight in hexaploid wheat. New Phytol. 2017, 215, 1026–1038. [Google Scholar] [CrossRef]
- Liu, S.T.; Liu, S.W.; Wang, M.; Wei, T.D.; Meng, C.; Wang, M.; Xia, G.M. A Wheat SIMILAR To RCD-ONE Gene Enhances Seedling Growth and Abiotic Stress Resistance by Modulating Redox Homeostasis and Maintaining Genomic Integrity. Plant Cell 2014, 26, 164–180. [Google Scholar] [CrossRef]
- Wang, W.Q.; Li, Q.X.; Tian, F.X.; Deng, Y.M.; Wang, W.L.; Wu, Y.Z.; Yang, J.J.; Wang, Y.; Hao, Q.Q.; Wang, W. Wheat NILs contrasting in grain size show different expansin expression, carbohydrate and nitrogen metabolism that are correlated with grain yield. Field Crop. Res. 2019, 241, 0378–4290. [Google Scholar] [CrossRef]
- Ma, Y.M.; Yang, C.; He, Y.; Tian, Z.H.; Li, J.X. Rice OVATE family protein 6 regulates plant development and confers resistance to drought and cold stresses. J. Exp. Bot. 2017, 68, 4885–4898. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, W.X.; Qin, M.Y.; Li, S.; Qiao, M.; Liu, Z.H.; Xiang, F.N. Drought tolerance conferred in Soybean (Glycine max. L) by GmMYB84, a novel R2R3-MYB transcription factor. Plant Cell Physiol. 2017, 58, 1764–1776. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Chen, L.; Wang, L.; Yang, Y.L.; Rao, Y.C.; Ren, D.Y.; Dai, L.P.; Gao, Y.H.; Zou, W.W.; Lu, X.L.; et al. A Nck-associated protein 1-like protein affects drought sensitivity by its involvement in leaf epidermal development and stomatal closure in rice. Plant J. 2019, 98, 884–897. [Google Scholar] [CrossRef]
- Matsuda, S.; Takano, S.; Sato, M.; Furukawa, K.; Nagasawa, H.; Yoshikawa, S.; Kasuga, J.; Tokuji, Y.; Yazaki, K.; Nakazono, M.; et al. Rice stomatal closure requires guard cell plasma membrane ATP-binding cassette transporter RCN1/OsABCG5. Mol. Plant 2016, 9, 417–427. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Tian, X.J.; Zhao, Q.Z.; Liu, Z.Q.; Li, X.F.; Ren, Y.K.; Tang, J.Q.; Fang, J.; Xu, Q.J.; Bu, Q.Y. The E3 ligase DROUGHT HYPERSENSITIVE negatively regulates cuticular wax biosynthesis by promoting the degradation of transcription factor ROC4 in rice. Plant Cell 2018, 30, 228–244. [Google Scholar] [CrossRef]
- Kan, L.; Liao, Q.C.; Chen, Z.P.; Wang, S.Y.; Ma, Y.F.; Su, Z.Y.; Zhang, L. Dynamic Transcriptomic and Metabolomic Analyses of Madhuca pasquieri (Dubard) H. J. Lam During the Post-germination Stages. Front. Plant Sci. 2021, 12, 731203. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. 2010. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhao, X.; Wu, J.; Shou, H.; Wang, W. The F-Box Protein TaFBA1 Positively Regulates Drought Resistance and Yield Traits in Wheat. Plants 2024, 13, 2588. https://doi.org/10.3390/plants13182588
Li Q, Zhao X, Wu J, Shou H, Wang W. The F-Box Protein TaFBA1 Positively Regulates Drought Resistance and Yield Traits in Wheat. Plants. 2024; 13(18):2588. https://doi.org/10.3390/plants13182588
Chicago/Turabian StyleLi, Qinxue, Xiaoyu Zhao, Jiajie Wu, Huixia Shou, and Wei Wang. 2024. "The F-Box Protein TaFBA1 Positively Regulates Drought Resistance and Yield Traits in Wheat" Plants 13, no. 18: 2588. https://doi.org/10.3390/plants13182588





