Effects of Salicylic Acid on Physiological Responses of Pepper Plants Pre-Subjected to Drought under Rehydration Conditions
Abstract
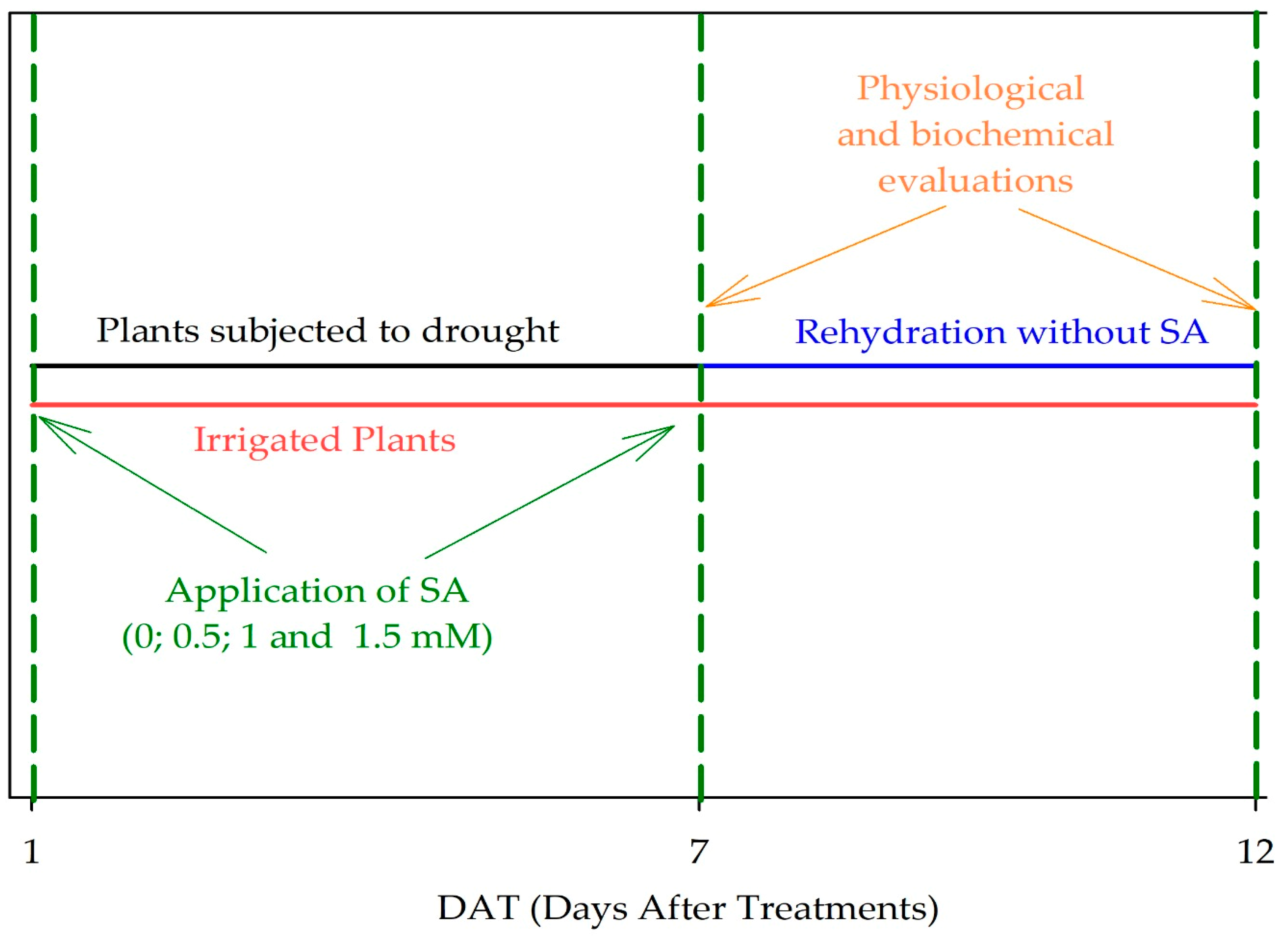
:1. Introduction
2. Results
2.1. Effects of Treatments on Relative Leaf Water Content, Plant Water Potential, Chlorophyll Content (a and b), and Carotenoids
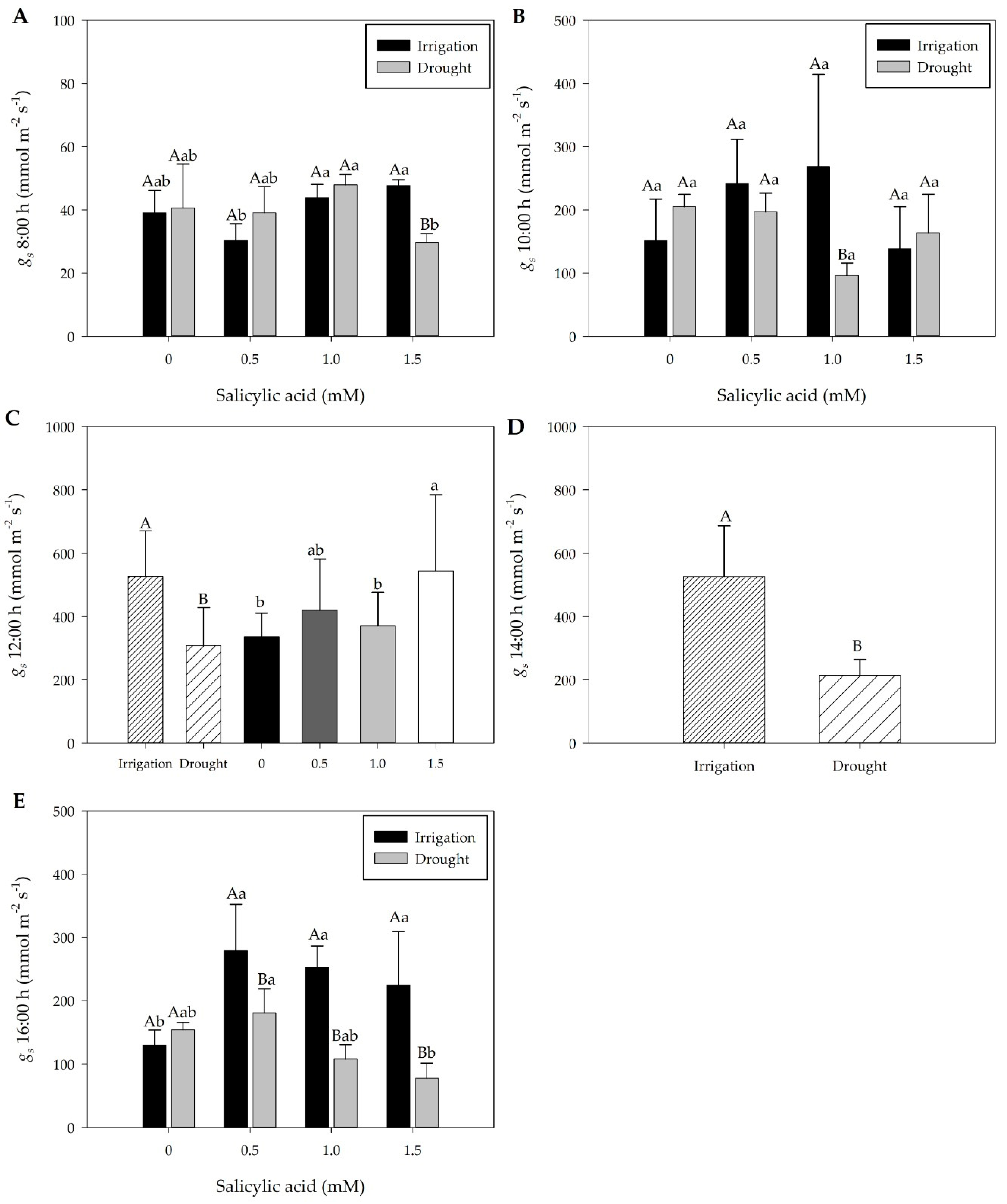
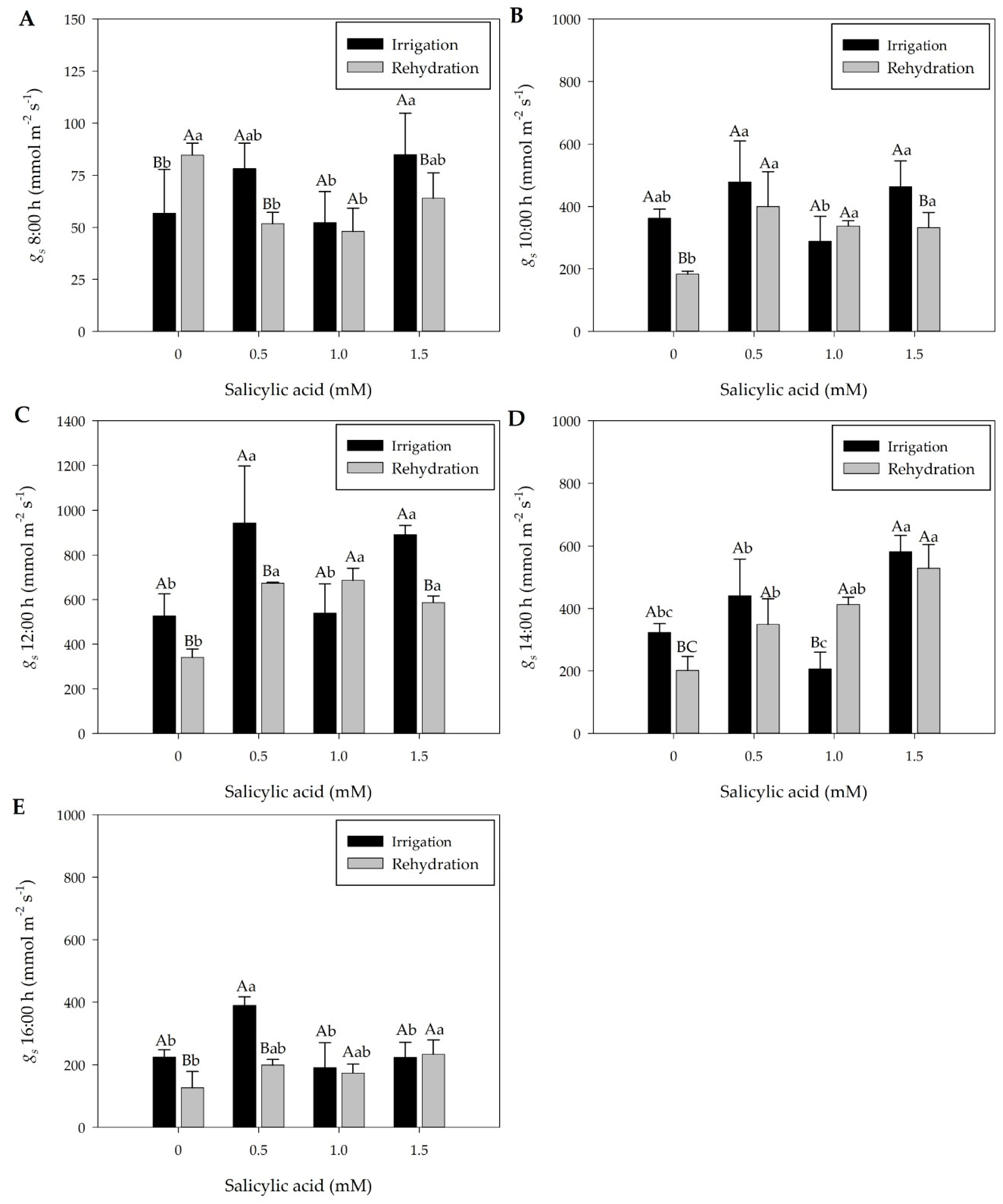
2.2. Effect of Treatments on Stomatal Conductance
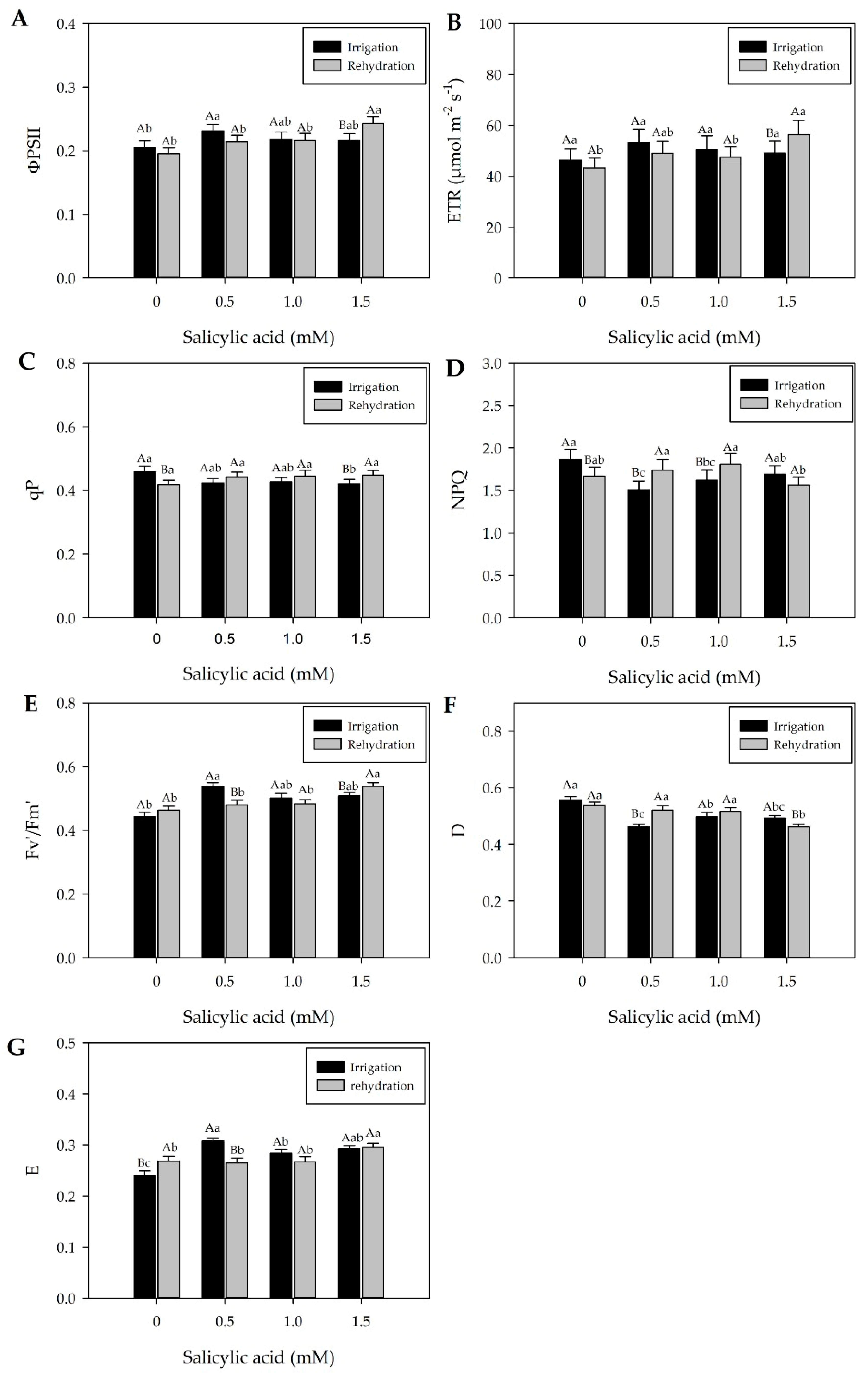
2.3. Effects of Treatments on Chlorophyll Fluorescence
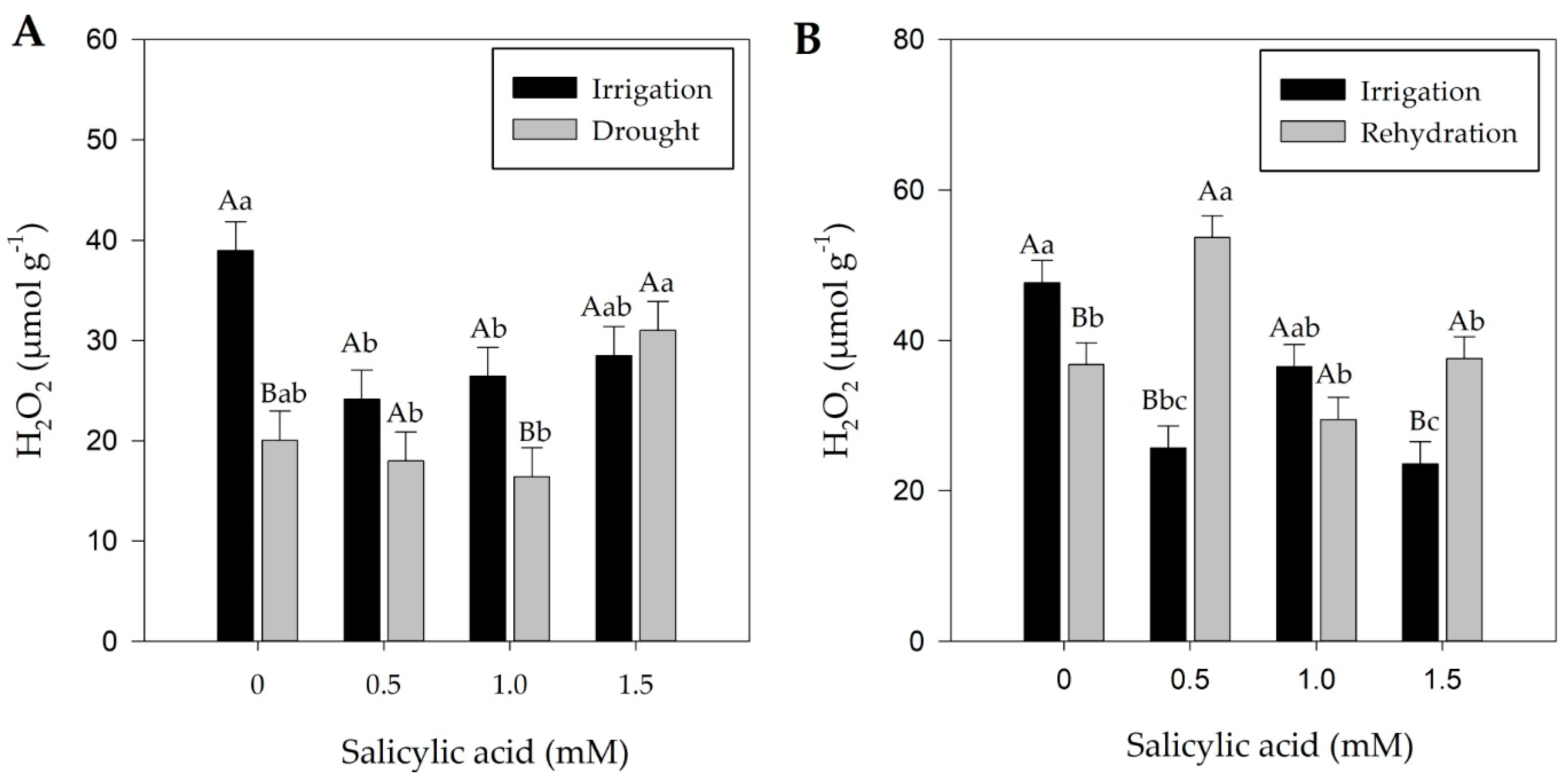
2.4. Effect of Treatments on Hydrogen Peroxide
3. Discussion
3.1. Effect of Drought and Rehydration on Irrigated Plants Cultivated without Salicylic Acid
3.2. Effect of Salicylic Acid on Mitigating the Effects of Drought and Rehydration Compared to Irrigated Conditions
4. Material and Methods
4.1. Experiment Location
4.2. Seedling Production, Soil Physical and Chemical Characteristics and Irrigation Monitoring
4.3. Water Regime
4.4. Foliar Application of Salicylic Acid and Assessments
4.5. Experimental Design
4.6. Leaf Relative Water Content (RWC)
4.7. Plant Water Potential (Ψw)
4.8. Chlorophylls a and b and Total Carotenoid Content
4.9. Stomatal Conductance
4.10. Chlorophyll a Fluorescence
4.11. Determination of the Hydrogen Peroxide (H2O2) Content
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laxa, M.; Michael Liebtha, M.; Telman, W.; Chibani, K.; Karl-Josef Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Namaki, A.; Ghahremani, Z.; Aelaei, M.; Barzegar, T.; Ranjabr, M.E. Morpho-physiological responses of asparagus accessions to drought stress under greenhouse condition. Gesunde Pflanz. 2022, 74, 925–934. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef]
- Iqbal, H.; Yaning, C.; Waqas, M.; Raza, S.T.; Shareef, M.; Ahmad, Z. Salinity and exogenous H2O2 improve gas exchange, osmoregulation, and antioxidant metabolism in quinoa under drought stress. Physiol. Plant. 2023, 175, 6.e14057. [Google Scholar] [CrossRef]
- Liu, X.; Chen, A.; Wang, Y.; Jin, G.; Zhang, Y.; Gu, L.; Li, C.; Shao, X.; Wang, K. Physiological and transcriptomic insights into adaptive responses of Seriphidium transiliense seedlings to drought stress. Environ. Exp. Bot. 2022, 194, 104736. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; He, Q.; Zhou, H. Stomatal limitations to photosynthesis and their critical Water conditions in different growth stages of maize under water stress. Agric. Water Manag. 2020, 241, 106330. [Google Scholar] [CrossRef]
- Nicolau, M.C.M.; Checchio, M.V.; de Moura Gonçalves, F.C.; Souza, L.A.; Gratão, P.L.; de Oliveira Cantão, F.R.; Carvalho, R.F. Interaction of the Selenium and Silicon with Hormones in Drought Stress Response. In Metals and Metalloids in Plant Signaling; Aftab, T., Ed.; Signaling and Communication in Plants; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the Role of Foliar Applied Salicylic Acid in Decreasing Chlorophyll Content to Reassess Photosystem II Photoprotection in Crop Plants. Int. J. Mol. Sci 2022, 23, 7038. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does it Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Roach, T.; Krieger-Liszkay, A. Regulation of Photosynthetic Electron Transport and Photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef]
- Boamah, S.; Ojangba, T.; Zhang, S.; Zhu, N.; Osei, R.; John Tiika, R.; Boakye, T.A.; Khurshid, A.; Inayat, R.; Effah, Z.; et al. Evaluation of salicylic acid (SA) signaling pathways and molecular markers in Trichoderma-treated plants under salinity and Fusarium stresses. A Review. Eur. J. Plant Pathol. 2023, 166, 259–274. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q. Exogenous application of salicylic acid via seed soaking improved growth and photosynthetic efficiency by maintaining stomatal organisation, redox homeostasis, and antioxidant defense system in tomato (Solanum lycopersicum L.). Acta Physiol. Plant. 2024, 46, 41. [Google Scholar] [CrossRef]
- Ahmad, R.; Manzoor, M.; Muhammad, H.M.D.; Altaf, M.A.; Shakoor, A. Exogenous melatonin spray enhances salinity tolerance in Zizyphus germplasm: A brief theory. Life 2023, 13, 493. [Google Scholar] [CrossRef]
- Feng, B.H.; Li, G.Y.; Islam, M.; Fu, W.M.; Zhou, Y.Q.; Chen, T.T.; Tao, L.X.; Fu, G.F. Strengthened antioxidant capacity improves photosynthesis by regulating stomatal aperture and ribulose-1,5-bisphosphate carboxylase/oxygenase activity. Plant Sci. 2020, 290, 110245. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 2318. [Google Scholar] [CrossRef]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in Roles of Salicylic Acid in Plant Tolerance Responses to Biotic and Abiotic Stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef]
- Pokotylo, I.; Kravets, V.; Ruelland, E. Salicylic acid binding proteins (SABPs): The hidden forefront of salicylic acid signalling. Int. J. Mol. Sci. 2019, 20, 4377. [Google Scholar] [CrossRef]
- Aires, E.S.; Ferraz, A.K.L.; Carvalho, B.L.; Teixeira, F.P.; Putti, F.F.; de Souza, E.P.; Rodrigues, J.D.; Ono, E.O. Foliar Application of Salicylic Acid to Mitigate Water Stress in Tomato. Plants 2022, 11, 1775. [Google Scholar] [CrossRef]
- Morato de Moraes, D.H.; Mesquita, M.; Magalhães Bueno, A.; Alves Flores, R.; Elias de Oliveira, H.F.; Raimundo de Lima, F.S.; de Mello Prado, R.; Battisti, R. Combined Effects of Induced Water Deficit and Foliar Application of Silicon on the Gas Exchange of Tomatoes for Processing. Agronomy 2020, 10, 1715. [Google Scholar] [CrossRef]
- Behnam, A.; Abbaspour, H.; Safipour Afshar, A.; Saeid Nematpour, F. Effect of salicylic acid on some of morphological and physiological traits of wheat (Triticum aestivum L.) under different levels of cadmium stress. Nativa 2018, 6, 594–599. [Google Scholar]
- Shemi, R.; Wang, R.; Gheith, E.S.M.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Melotto, M.; Zhang, L.; Oblessuc, P.R.; He, S.Y. Stomatal defense a decade later. Plant Physiol. 2017, 174, 561–571. [Google Scholar] [CrossRef]
- Alam, P.; Balawi, T.A.; Faizan, M. Salicylic Acid’s Impact on Growth, Photosynthesis, and Antioxidant Enzyme Activity of Triticum aestivum When Exposed to Salt. Molecules 2023, 28, 100. [Google Scholar] [CrossRef]
- Batista, V.C.V.; Pereira, I.M.C.; de Oliveira Paula-Marinho, S.; Canuto, K.M.; Pereira, R.D.C.A.; Rodrigues, T.H.S.; de Menezes Daloso, D.; Gomes-Filho, E.; de Carvalho, H.H. Salicylic acid modulates primary and volatile metabolites to alleviate salt stress-induced photosynthesis impairment on medicinal plant Egletes viscosa. Environ. Exp. Bot. 2019, 167, e103870. [Google Scholar] [CrossRef]
- Yang, H.; Fang, R.; Luo, L.; Yang, W.; Huang, Q.; Yang, C.; Hui, W.; Gong, W.; Wang, J. Uncovering the mechanisms of salicylic acid-mediated abiotic stress tolerance in horticultural crops. Front. Plant Sci. 2023, 14, 1226041. [Google Scholar] [CrossRef]
- Elvwan, M.W.M.; Hamahyomy, M.A.M. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 2009, 122, 521–526. [Google Scholar] [CrossRef]
- Admassie, M.; Woldehawariat, Y.; Alemu, T.; Gonzalez, E.; Jimenez, J.F. The role of plant growth promoting bacteria in alleviating drought stress on pepper plants. Agric Water Manag. 2022, 272, 107831. [Google Scholar] [CrossRef]
- Sanatombi, K.; Rajkumari, S. Effect of processing on quality of pepper: A review. Food Rev. Int. 2020, 36, 626–643. [Google Scholar] [CrossRef]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, anti-obesity, nutritional and Other beneficial effects of different chili pepper: A review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, C.B.; Ren, R.M.; Jiang, J.H. Salicylic acid had the potential to enhance tolerance in horticultural crops against abiotic stress. Front. Plant Sci. 2023, 14, 1141918. [Google Scholar] [CrossRef]
- Ennab, H.A.; El-Shemy, M.A.; Alam-Eldein, S.M. Salicylic acid and putrescine to reduce post-harvest storage problems and maintain quality of mandarin fruit. Agron. J. 2020, 10, 115–130. [Google Scholar] [CrossRef]
- Nezamdoost, D.; Ghahremani, Z.; Baba Akbari, M.; Barzegar, T.; Ranjbar, M.E. Irrigation with water enriched with seaweed extract to overcome effects of salinity in ‘New red fire’ leafy lettuce cultivation. Int. J. Veg. Sci. 2023, 29, 128–144. [Google Scholar] [CrossRef]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic Acid, a Multifaceted Hormone, Combats Abiotic Stresses in Plants. Life 2022, 12, 886. [Google Scholar] [CrossRef]
- Chadee, A.; Alber, N.A.; Dahal, K.; Vanlerberghe, G.C. The Complementary Roles of Chloroplast Cyclic Electron Transport and Mitochondrial Alternative Oxidase to Ensure Photosynthetic Performance. Front. Plant Sci. 2021, 12, 748204. [Google Scholar] [CrossRef]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. Singlet Oxygen in Plants: Generation, Detection, and Signaling Roles. Int. J. Mol. Sci. 2020, 21, 3237. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Me, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Application of chlorophyll fluorescence can improve crop production strategies: And examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Gonçalves, F.C.D.M.; Parreiras, N.D.S.; Campos, F.G.; Mantoan, L.P.B.; Boaro, C.S.F. Exogenous salicylic acid modifies gas exchange and biomass production of Mentha × piperita L. Aust. J. Crop Sci. 2020, 1, 98–107. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Alizadeh, B.; Barzegar, T.; Nikbakht, J.; Ranjbar, M.E.; Nezamdoost, D. The mechanism of enhancing drought tolerance threshold of pepper plant treated with putrescine and salicylic acid. Plant Stress 2023, 9, 100199. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Escobar, A.L.; Bravo, L.A. Salicylic Acid Improves Antioxidant Defense System and Photosynthetic Performance in Aristotelia chilensis Plants Subjected to Moderate Drought Stress. Plants 2022, 11, 639. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Shan, L.; Xu, Y.; Wu, D.; Hu, J.; Yu, T.; Dang, C.; Fang, Y.; Zhang, X.; Tian, Q.; Xue, D. Effects of salicylic acid on growth, physiology, and gene expression in rice seedlings under salt and drought stress. Plant Stress 2024, 11, 100413. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, S.; Jalal, F.; Khan, M.A.; Imtiaz, M.; Said, F.; Ismail, M.; Khan, S.; Ali, H.M.; Hatamleh, A.A.; et al. Salicylic acid-mitigates abiotic stress tolerance via altering defense mechanisms in Brassica napus (L. ). Front. Plant Sci. 2023, 14, 1187260. [Google Scholar] [CrossRef]
- Waseem, M.; Athar, H.U.R.; Ashrafi, M. Effect of salicylic acid applied through rooting medium on drought tolerance of wheat. Pak. J. Bot. 2006, 38, 1127–1136. [Google Scholar]
- Ghahremani, Z.; Mikaealzadeh, M.; Barzegar, T.; Ranjabr, M.E. Foliar application of ascorbic acid and gamma aminobutyric acid can improve important properties of deficit irrigated cucumber plants (Cucumis sativus cv. Us). Gesunde Pflanz. 2021, 73, 77–84. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Poor, P.; Janda, T. Salicylic Acid: A Versatile Signaling Molecule in Plants. J. Plant Growth Regul. 2022, 41, 1887–1890. [Google Scholar] [CrossRef]
- Poo’r, P.; Tari, I. Regulation of stomatal movement and photosynthetic activity in guard cells of tomato abaxial epidermal peels by salicylic acid. Funct. Plant Biol. 2012, 39, 1028–1037. [Google Scholar] [CrossRef]
- Franzisky, B.L.; Sölter, J.; Xue, C.; Harter, K.; Stahl, M.; Geilfus, C.M. In planta exploitation of leaf apoplastic compounds: A window of opportunity for spatiotemporal studies of apoplastic metabolites, hormones and physiology. Biorxiv 2023, 4, 2023-04. [Google Scholar]
- Lauterberg, M.; Tschiersch, H.; Zhao, Y.; Kuhlmann, M.; Mücke, I.; Papa, R.; Bitocchi, E.; Neumann, K. Implementation of theoretical non-photochemical quenching (NPQ(T)) to investigate NPQ of chickpea under drought stress with High-throughput Phenotyping. Scientifc Reports. 2024, 14, 13970. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Souza, G.M.; Manzatto, A.G.; Machado, E.C.; Oliveira, R.F. Chlorophyll fluorescence of tropical tree species in a semi-deciduous forest gap. Rev. Árvore 2004, 28, 21–27. [Google Scholar] [CrossRef]
- Vazirimehr, M.; Ganjali, H.; Rigi, K.; Keshtehgar, A. Effect of seed priming on quantitative traits corn. Int. J. Plant Sci. 2014, 4, 134–140. [Google Scholar]
- Sahu, G.K.; Kar, M.; Sabat, S.C. Alteration in phosphate uptake potential of wheat plants co-cultivated with salicylic acid. J Plant Physiol. 2010, 167, 326–328. [Google Scholar] [CrossRef]
- Ullah, F.; Saqib, S.; Khan, W.; Ayaz, A.; Batool, A.; Wang, W.Y.; Xiong, Y.C. The multifaceted role of sodium nitroprusside in plants: Crosstalk with phytohormones under normal and stressful conditions. Plant Growth Regul. 2024, 103, 453–470. [Google Scholar] [CrossRef]
- Varone, L.; Ribas-Carbo, M.; Carnoda, C.; Gallé, A.; Medrano, H.; Gratani, L.; Flexas, J. Stomatal and nonstomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Bot. 2012, 75, 235–247. [Google Scholar] [CrossRef]
- Thameur, A.; Lachihe, B.; Ferchichi, A. Drought effect on growth, gas exchange and yield, in two strains of local barley Ardhaoui, under water deficit conditions in southern Tunisia. J. Environ. Manage. 2012, 113, 495–500. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Technol. 2015, 52, 3607–3616. [Google Scholar] [CrossRef]
- Mantoan, L.P.B.; Ferreira, G.; Boaro, C.S.F. Chlorophyll a fluorescence in Annona emarginata (Schltdl.) H. Rainer plants subjected to water stress and after rehydration. Sci. Hortic. 2015, 184, 23–30. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in water relations of cotton plant. In: The field measurament of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Hemingsen, E.A.; Bradstreet, E.D. Hydrostatic pressure and osmotic potencials in leaves of mangroves and some other plants. Proc. Natl. Acad. Sci. USA 1965, 51, 119–125. [Google Scholar]
- Turner, N.C. Techniques and experimental approaches for the measure ment of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Moran, R. Formulae for Determination of Chlorophyllous Pigments Extracted with N,N-Dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids—Extraction, Isolation and Purification. Current Protocols in Food Analytical Chemistry (CPFA), (Supplement 1), Unit F4.2.1-F4.2.6; John Wiley: New York, NY, USA, 2001. [Google Scholar]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 1, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induce absorbance and photosynthesis in Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice-Hall: Upper Saddle River, NJ, USA, 2010; p. 944. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes.pt: Pacote Experimental Designs (Portuguese). R Package Version 1.2.0. 2018. Available online: https://CRAN.R-project.org/package=ExpDes.pt (accessed on 1 February 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, F.C.d.M.; Mantoan, L.P.B.; Corrêa, C.V.; Parreiras, N.d.S.; de Almeida, L.F.R.; Ono, E.O.; Rodrigues, J.D.; Prado, R.d.M.; Boaro, C.S.F. Effects of Salicylic Acid on Physiological Responses of Pepper Plants Pre-Subjected to Drought under Rehydration Conditions. Plants 2024, 13, 2805. https://doi.org/10.3390/plants13192805
Gonçalves FCdM, Mantoan LPB, Corrêa CV, Parreiras NdS, de Almeida LFR, Ono EO, Rodrigues JD, Prado RdM, Boaro CSF. Effects of Salicylic Acid on Physiological Responses of Pepper Plants Pre-Subjected to Drought under Rehydration Conditions. Plants. 2024; 13(19):2805. https://doi.org/10.3390/plants13192805
Chicago/Turabian StyleGonçalves, Fabrício Custódio de Moura, Luís Paulo Benetti Mantoan, Carla Verônica Corrêa, Nathália de Souza Parreiras, Luiz Fernando Rolim de Almeida, Elizabeth Orika Ono, João Domingos Rodrigues, Renato de Mello Prado, and Carmen Sílvia Fernandes Boaro. 2024. "Effects of Salicylic Acid on Physiological Responses of Pepper Plants Pre-Subjected to Drought under Rehydration Conditions" Plants 13, no. 19: 2805. https://doi.org/10.3390/plants13192805






