Physiological and Transcriptomic Analyses Reveal the Mechanisms Underlying Methyl Jasmonate-Induced Mannitol Stress Resistance in Banana
Abstract
1. Introduction
2. Results
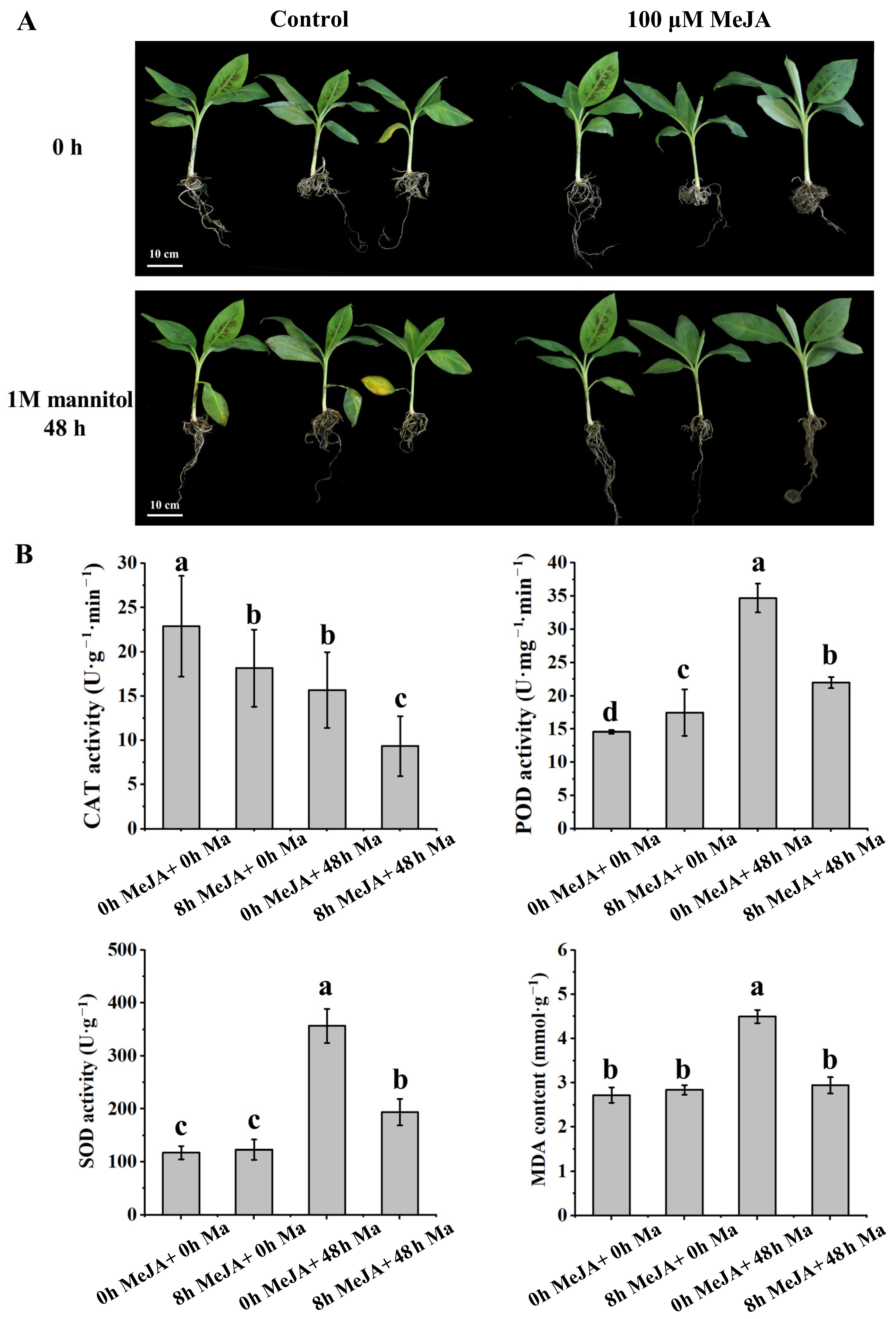
2.1. MeJA Treatment Improved Abiotic Stress Resistance in Banana
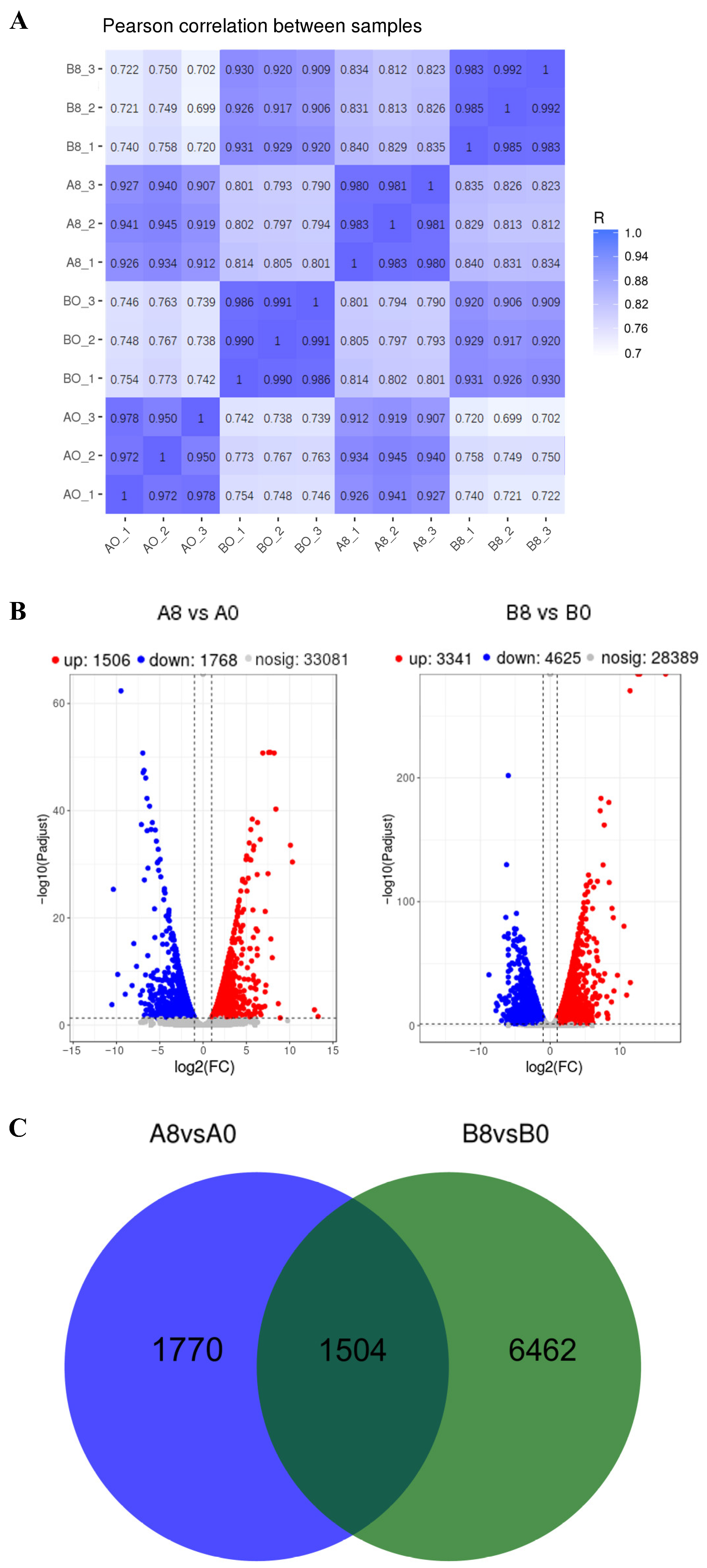
2.2. Sample Preparation and RNA Quality Assessment
2.3. Identification and Analysis of Differentially Expressed Genes
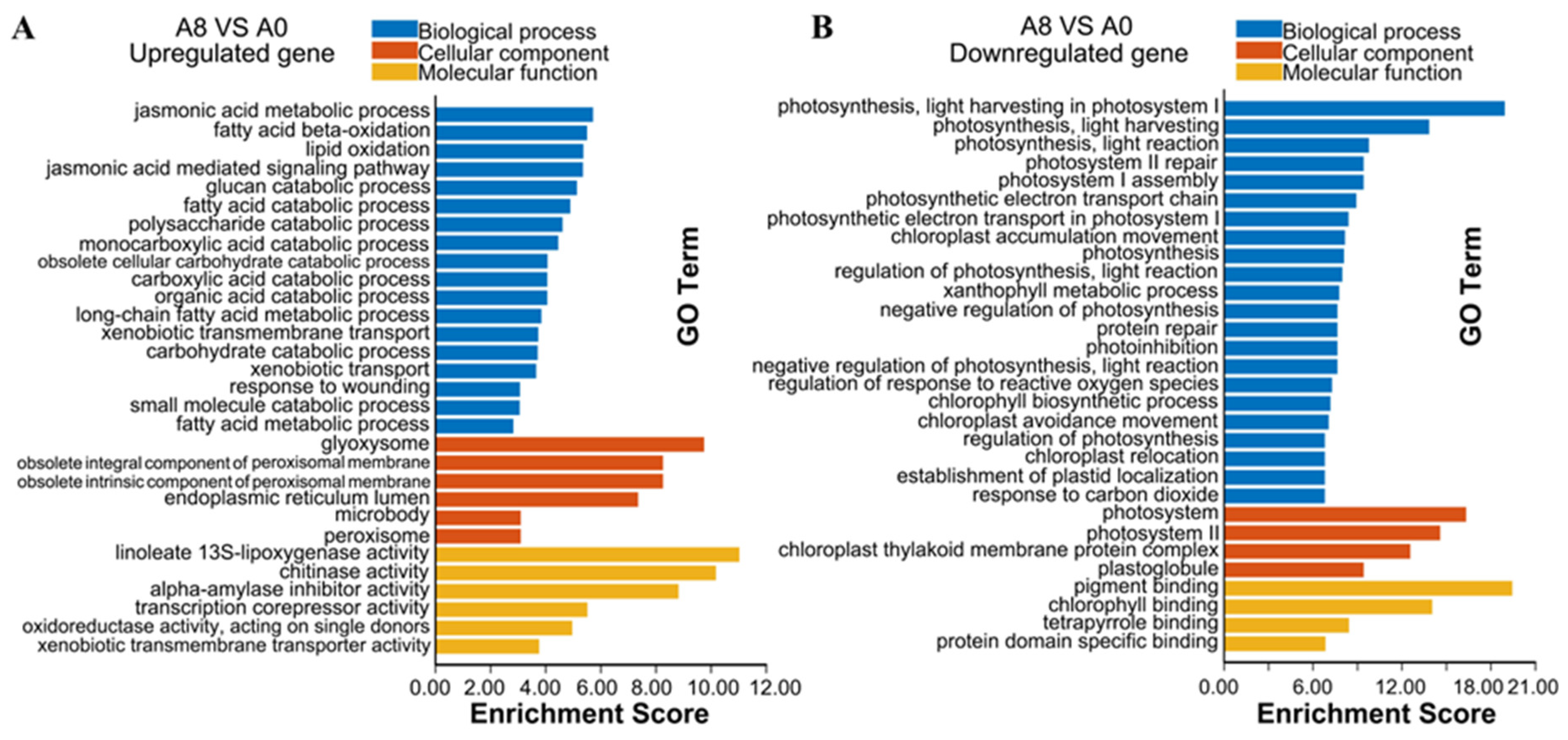
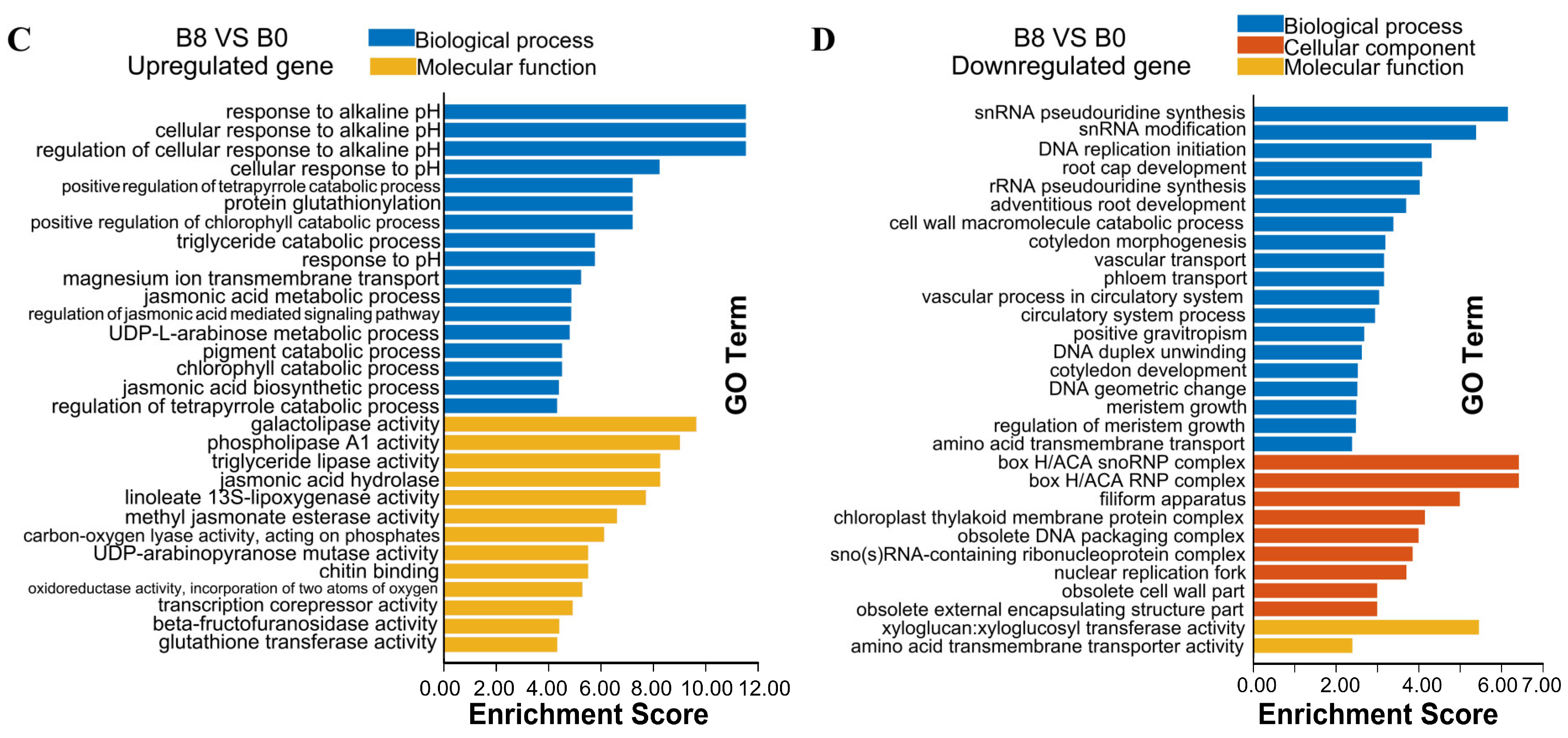
2.4. Gene Ontology Enrichment Analysis of the DEGs
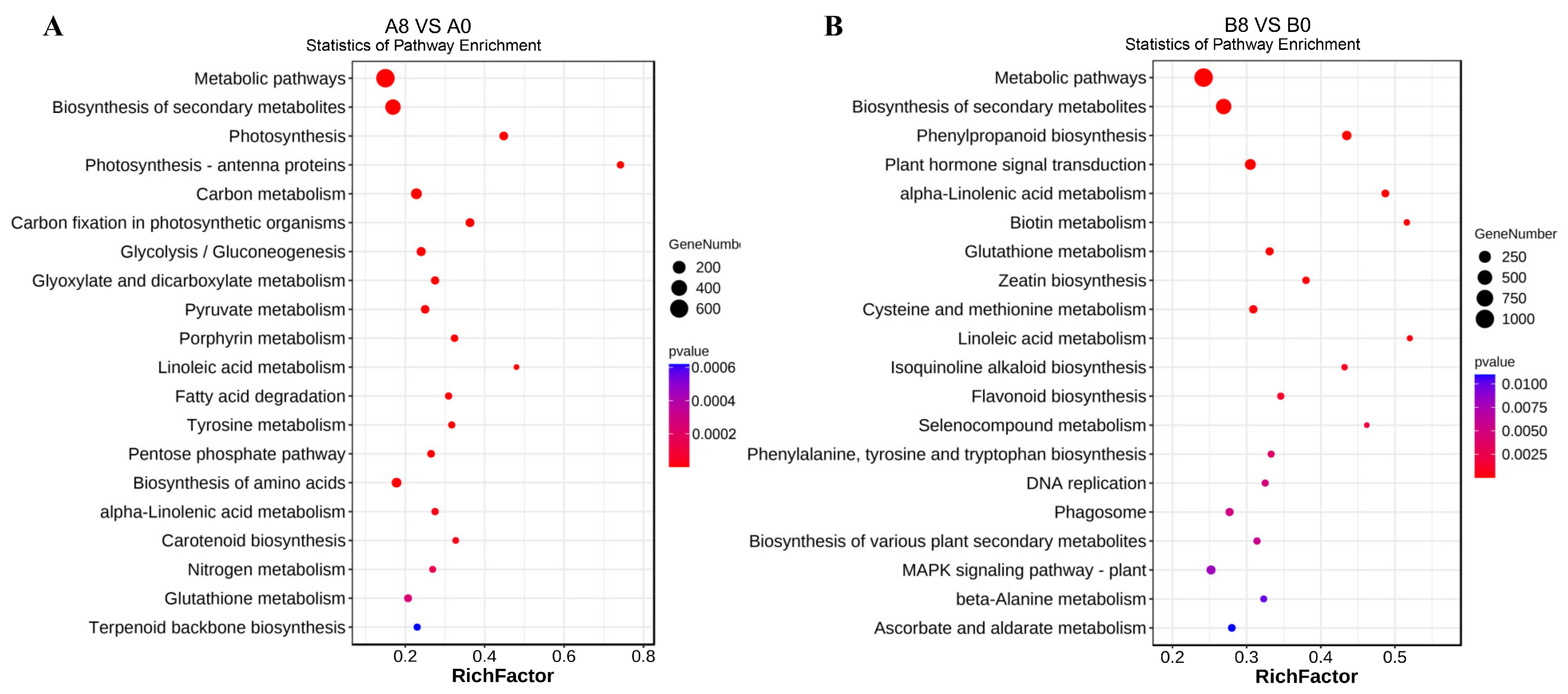
2.5. Kyoto Encyclopedia of Genes and Genomes Enrichment Analysis of DEGs
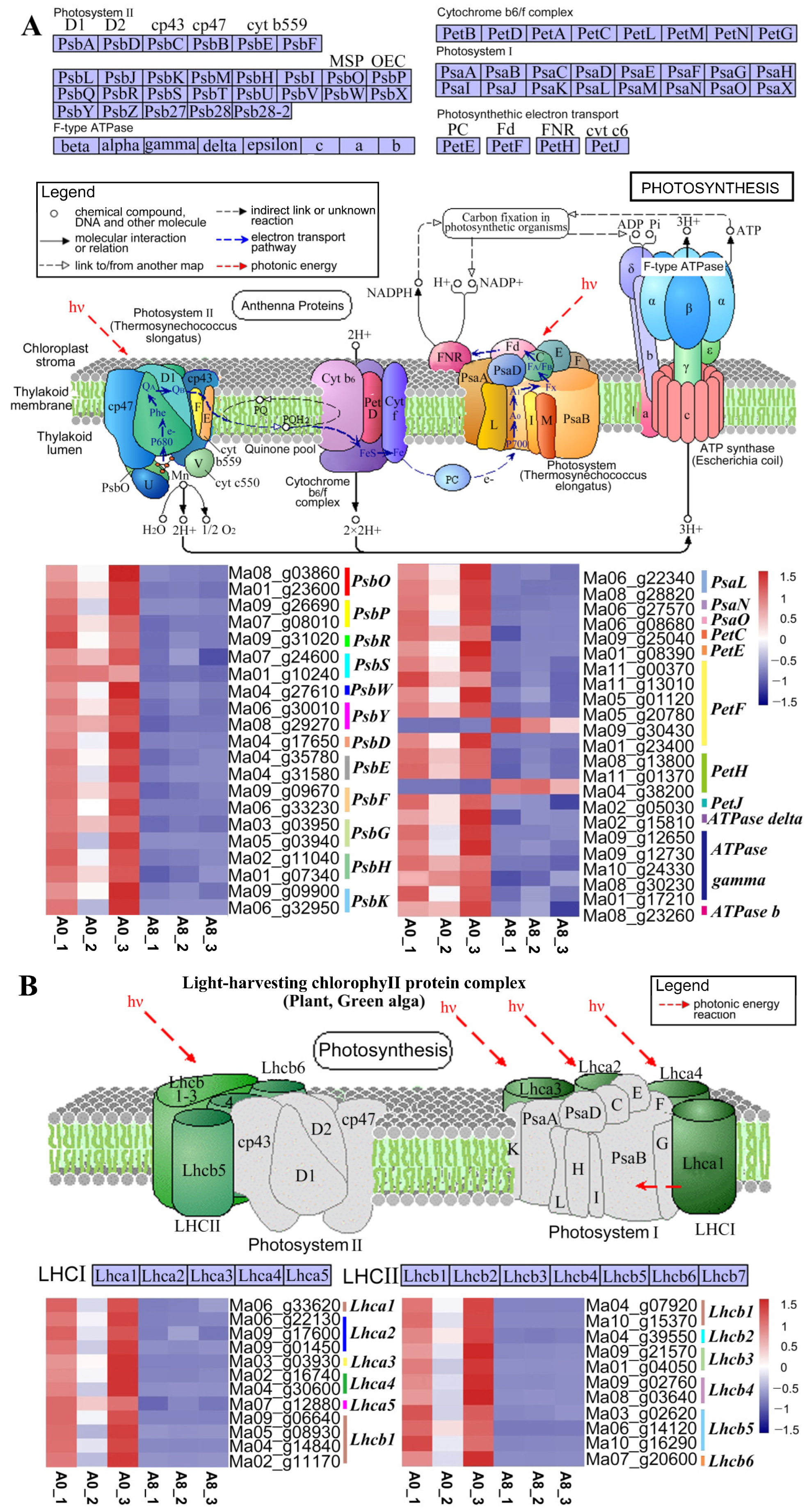
2.6. Regulatory Mechanisms in Leaves Altered upon MeJA Treatment
2.7. Regulatory Mechanisms in Roots Altered upon MeJA Treatment
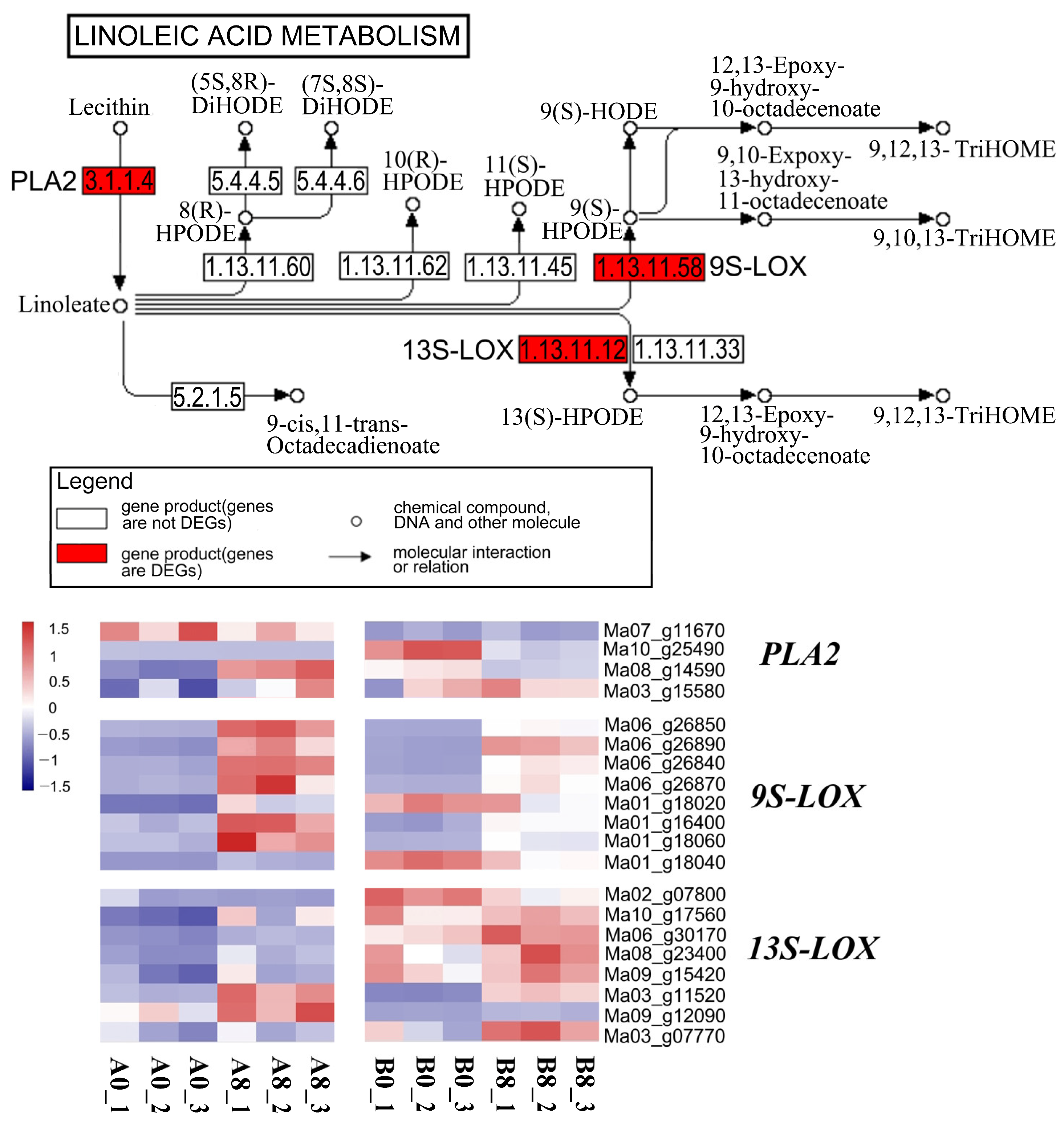
2.8. Linoleic Acid Metabolism Showed Tissue-Specific Enrichment
2.9. Analysis of the Promoter Region of the Genes Involved in Linoleic Acid Metabolism
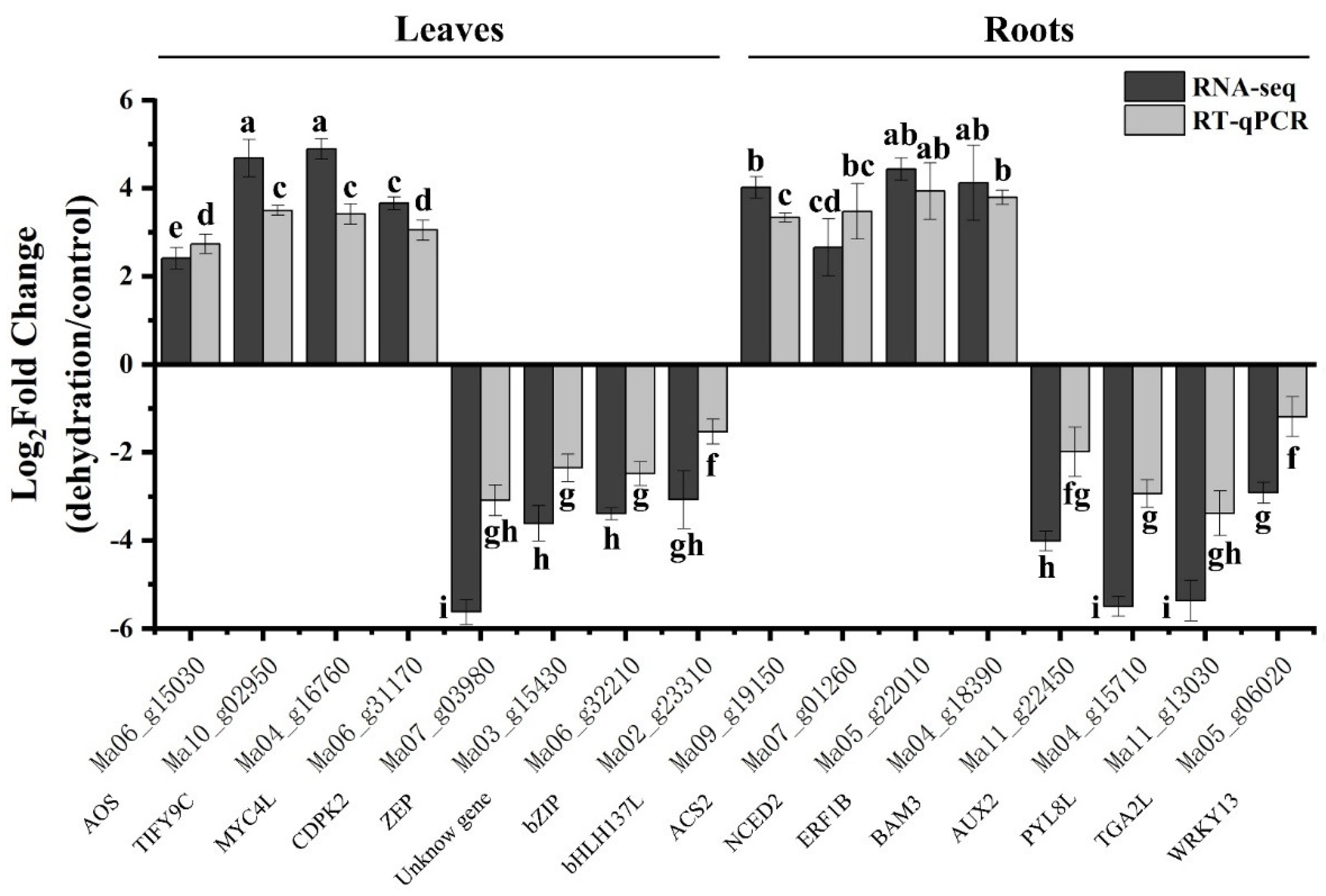
2.10. Verification of the Expression Patterns of DEGs
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Measurement of CAT, SOD, and POD Activity and MDA Content
4.3. RNA-Seq and Expression Analysis
4.4. GO and KEGG Enrichment Analysis of DEGs
4.5. Promoter Sequence Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Li, M.; Jiang, Y.; Duan, X. Genome-Wide Identification, Characterization and Expression Profile of Glutaredoxin Gene Family in Relation to Fruit Ripening and Response to Abiotic and Biotic Stresses in Banana (Musa acuminata). Int. J. Biol. Macromol. 2021, 170, 636–651. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, W.; Liu, J.; Zhang, J.; Jia, C.; Miao, H.; Xu, B.; Jin, Z. A Banana Aquaporin Gene, MaPIP1;1, Is Involved in Tolerance to Drought and Salt Stresses. BMC Plant Biol. 2014, 14, 59. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, W.; Liu, J.H.; Song, S.; Hou, X.W.; Jia, C.H.; Li, J.Y.; Miao, H.X.; Wang, Z.; Tie, W.W.; et al. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol. Biochem. 2020, 147, 66–76. [Google Scholar] [CrossRef]
- van Asten, P.J.A.; Fermont, A.M.; Taulya, G. Drought Is a Major Yield Loss Factor for Rainfed East African Highland Banana. Agric. Water Manag. 2011, 98, 541–552. [Google Scholar] [CrossRef]
- Vanhove, A.-C. Screening the Banana Biodiversity for Drought Tolerance: Can an in Vitro Growth Model and Proteomics Be Used as a Tool to Discover Tolerant Varieties and Understand Homeostasis. Front. Plant Sci. 2012, 3, 176. [Google Scholar] [CrossRef]
- Ravi, I. Phenotyping Bananas for Drought Resistance. Front. Physiol. 2013, 4, 9. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-Responsive Mechanisms in Plant Leaves Revealed by Proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Li, S.; Niinemets, Ü. Methyl Jasmonate-Induced Emission of Biogenic Volatiles Is Biphasic in Cucumber: A High-Resolution Analysis of Dose Dependence. J. Exp. Bot. 2017, 68, 4679–4694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ye, J.; Rasulov, B.; Niinemets, Ü. Role of Stomatal Conductance in Modifying the Dose Response of Stress-Volatile Emissions in Methyl Jasmonate Treated Leaves of Cucumber (Cucumis sativa). Int. J. Mol. Sci. 2020, 21, 1018. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl Jasmonate Mediates Melatonin-Induced Cold Tolerance of Grafted Watermelon Plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, J.; Li, P.; Yan, Z.; Gao, L.; Wang, Q.; Jiang, A. Effect of Methyl Jasmonate on the Quality of Harvested Broccoli after Simulated Transport. Food Chem. 2020, 319, 126561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-L.; Wang, J.-N.; Shan, W.; Fan, J.-G.; Kuang, J.-F.; Wu, K.-Q.; Li, X.-P.; Chen, W.-X.; He, F.-Y.; Chen, J.-Y.; et al. Induction of Jasmonate Signalling Regulators MaMYC2s and Their Physical Interactions with MaICE1 in Methyl Jasmonate-Induced Chilling Tolerance in Banana Fruit. Plant Cell Environ. 2012, 36, 30–51. [Google Scholar] [CrossRef]
- Peng, H.; Shan, W.; Kuang, J.; Lu, W.; Chen, J. Molecular Characterization of Cold-Responsive Basic Helix-Loop-Helix Transcription Factors MabHLHs That Interact with MaICE1 in Banana Fruit. Planta 2013, 238, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, H.; Wu, J.; Wang, B.; Tian, N.; Liu, J.; Sun, X.; Wu, H.; Huang, Y.; Lü, P.; et al. Genome-Wide Identification and Expression Pattern Analysis of Lipoxygenase Gene Family in Banana. Sci. Rep. 2021, 11, 9948. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.-J.; Choi, Y.D. Methyl Jasmonate as a Vital Substance in Plants. Trends Genet. 2003, 19, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Tamogami, S.; Rakwal, R.; Agrawal, G.K. Interplant Communication: Airborne Methyl Jasmonate Is Essentially Converted into JA and JA-Ile Activating Jasmonate Signaling Pathway and VOCs Emission. Biochem. Biophys. Res. Commun. 2008, 376, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, C.; Qi, D.; Lv, H.; Yang, T.; Peng, Q.; Chen, Z.; Lin, Z. Transcriptional Responses and Flavor Volatiles Biosynthesis in Methyl Jasmonate-Treated Tea Leaves. BMC Plant Biol. 2015, 15, 1–22. [Google Scholar] [CrossRef]
- Muralla, R.; Chen, E.; Sweeney, C.; Gray, J.A.; Dickerman, A.; Nikolau, B.J.; Meinke, D. A Bifunctional Locus (BIO3-BIO1) Required for Biotin Biosynthesis in Arabidopsis. Plant Physiol. 2007, 146, 60–73. [Google Scholar] [CrossRef]
- Cobessi, D.; Dumas, R.; Pautre, V.; Meinguet, C.; Ferrer, J.-L.; Alban, C. Biochemical and Structural Characterization of the Arabidopsis Bifunctional Enzyme Dethiobiotin Synthetase–Diaminopelargonic Acid Aminotransferase: Evidence for Substrate Channeling in Biotin Synthesis. Plant Cell 2012, 24, 1608–1625. [Google Scholar] [CrossRef]
- Tanabe, Y.; Maruyama, J.; Yamaoka, S.; Yahagi, D.; Matsuo, I.; Tsutsumi, N.; Kitamoto, K. Peroxisomes Are Involved in Biotin Biosynthesis in Aspergillus and Arabidopsis. J. Biol. Chem. 2011, 286, 30455–30461. [Google Scholar] [CrossRef] [PubMed]
- Arnal, N.; Alban, C.; Quadrado, M.; Grandjean, O.; Mireau, H. The Arabidopsis Bio2 Protein Requires Mitochondrial Targeting for Activity. Plant Mol. Biol. 2006, 62, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Picciocchi, A.; Douce, R.; Alban, C. The Plant Biotin Synthase Reaction. J. Biol. Chem. 2003, 278, 24966–24975. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, Z.A.; Khalafaallah, A.A.; Abdallah, M.M. Impact of Methyl Jasmonate on Antioxidant Activity and Some Biochemical Aspects of Maize Plant Grown under Water Stress Condition. Agric. Sci. 2014, 05, 1077–1088. [Google Scholar] [CrossRef]
- Wang, G.; Hu, C.; Zhou, J.; Liu, Y.; Cai, J.; Pan, C.; Wang, Y.; Wu, X.; Shi, K.; Xia, X.; et al. Systemic Root-Shoot Signaling Drives Jasmonate-Based Root Defense against Nematodes. Curr. Biol. 2019, 29, 3430–3438.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, J.; Yao, L.; Lu, Y. Enhanced Production of Flavonoids by Methyl Jasmonate Elicitation in Cell Suspension Culture of Hypericum Perforatum. Bioresour. Bioprocess. 2015, 2, 5–13. [Google Scholar] [CrossRef]
- Walia, H.; Wilson, C.; Wahid, A.; Condamine, P.; Cui, X.; Close, T.J. Expression Analysis of Barley (Hordeum Vulgare L.) during Salinity Stress. Funct. Integr. Genom. 2006, 6, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Hamayun, M.; Lee, S.-K.; Lee, I.-J. Methyl Jasmonate Alleviated Salinity Stress in Soybean. J. Crop Sci. Biotechnol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Sembdner, G.; Parthier, B. The biochemistry and the physiological and molecular actions of jasmonates. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 569–589. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in Plants: A New Light on Photosystem II Damage. Trends. Plant. Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Photo-Oxidative Stress during Leaf, Flower and Fruit Development. Plant Physiol. 2017, 176, 1004–1014. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pang, Q.; Dai, S.; Wang, Y.; Chen, S.; Yan, X. Proteomic Identification of Differentially Expressed Proteins in Arabidopsis in Response to Methyl Jasmonate. J. Plant Physiol. 2011, 168, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.G.D.B.; Reis, A.R. dos Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses against Abiotic Stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y.; Yu, Y.; Liu, H.; Rensing, C.; Wu, Z.; et al. Underlying Mechanisms Responsible for Restriction of Uptake and Translocation of Heavy Metals (Metalloids) by Selenium via Root Application in Plants. J. Hazard. Mater. 2021, 402, 123570. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, D.; Van Daele, J.; Storozhenko, S.; Stove, C.; Lambert, W.; Van Der Straeten, D. Rice Folate Enhancement through Metabolic Engineering Has an Impact on Rice Seed Metabolism, but Does Not Affect the Expression of the Endogenous Folate Biosynthesis Genes. Plant Mol. Biol. 2013, 83, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, G.; Frelin, O.; Roje, S.; Ellens, K.W.; Ali, K.; Guan, J.-C.; Garrett, T.J.; de Crécy-Lagard, V.; Gregory, J.F.; McCarty, D.R.; et al. Identification and Characterization of the Missing Pyrimidine Reductase in the Plant Riboflavin Biosynthesis Pathway. Plant Physiol. 2012, 161, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-Y.; Liao, J.-C.; Wang, H.-T.; Hung, T.-H.; Tseng, C.-C.; Chung, T.-Y.; Hsieh, M.-H. The Arabidopsis Thiamin-Deficient Mutant Pale Green1 Lacks Thiamin Monophosphate Phosphatase of the Vitamin B1 Biosynthesis Pathway. Plant J. 2017, 91, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Chun, S.C. Vitamin B6 Biosynthetic Genes Expression and Antioxidant Enzyme Properties in Tomato against, Erwinia Carotovora Subsp. Carotovora. Int. J. Biol. Macromol. 2018, 116, 31–36. [Google Scholar] [CrossRef]
- De Lepeleire, J.; Strobbe, S.; Verstraete, J.; Blancquaert, D.; Ambach, L.; Visser, R.G.F.; Stove, C.; Van Der Straeten, D. Folate Biofortification of Potato by Tuber-Specific Expression of Four Folate Biosynthesis Genes. Mol. Plant 2018, 11, 175–188. [Google Scholar] [CrossRef]
- Nan, N.; Wang, J.; Shi, Y.; Qian, Y.; Jiang, L.; Huang, S.; Liu, Y.; Wu, Y.; Liu, B.; Xu, Z. Rice Plastidial NAD -dependent Malate Dehydrogenase 1 Negatively Regulates Salt Stress Response by Reducing the Vitamin B6 Content. Plant Biotechnol. J. 2019, 18, 172–184. [Google Scholar] [CrossRef]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, J.; Jbara, M. Vitamins for Enhancing Plant Resistance. Planta 2016, 244, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Beaudoin, G.A.; McCarty, D.R.; Gregory, J.F. Does Abiotic Stress Cause Functional B Vitamin Deficiency in Plants? Plant Physiol. 2016, 172, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.A.; Orsomando, G.; Mehrshahi, P.; Lara-Núñez, A.; Bennett, M.J.; Gregory III, J.F.; Hanson, A.D. A Central Role for Gamma-Glutamyl Hydrolases in Plant Folate Homeostasis. Plant J. 2010, 64, 256–266. [Google Scholar] [CrossRef]
- Marbaix, A.Y.; Noël, G.; Detroux, A.M.; Vertommen, D.; Van Schaftingen, E.; Linster, C.L. Extremely Conserved ATP- or ADP-Dependent Enzymatic System for Nicotinamide Nucleotide Repair. J. Biol. Chem. 2011, 286, 41246–41252. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.P.; Moieni, A.; Hiraga, S.; Komatsu, S. Organ-Specific Proteomic Analysis of Drought-Stressed Soybean Seedlings. J. Proteom. 2012, 75, 1906–1923. [Google Scholar] [CrossRef] [PubMed]
- Piedrafita, G.; Keller, M.; Ralser, M. The Impact of Non-Enzymatic Reactions and Enzyme Promiscuity on Cellular Metabolism during (Oxidative) Stress Conditions. Biomolecules 2015, 5, 2101–2122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Ye, X.; Liu, H.; Takano, T.; Tsugama, D.; Liu, S.; Bu, Y. Biotin Plays an Important Role in Arabidopsis Thaliana Seedlings under Carbonate Stress. Plant. Sci. 2020, 300 Pt 1, 110639. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pang, J.; Zhang, F.; Sun, L.; Yang, L.; Zhao, Y.; Yang, Y.; Wang, Y.; Siddique, K.H.M. Integrated Transcriptomics and Metabolomics Analysis to Characterize Alkali Stress Responses in Canola (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 605–620. [Google Scholar] [CrossRef]
- Editors, T.P.O. Retraction: Potassium Fertilization Improves Growth, Yield and Seed Quality of Sunflower (Helianthus annuus L.) under Drought Stress at Different Growth Stages. PLoS ONE 2022, 17, e0272194. [Google Scholar] [CrossRef]
- Farmaki, T.; Sanmartin, M.; Jimenez, P.; Paneque, M.; Sanz, C.; Vancanneyt, G.; Leon, J.; Sanchez-Serrano, J.J. Differential Distribution of the Lipoxygenase Pathway Enzymes within Potato Chloroplasts. J. Exp. Bot. 2006, 58, 555–568. [Google Scholar] [CrossRef]
- Vicente, J.; Cascón, T.; Vicedo, B.; García-Agustín, P.; Hamberg, M.; Castresana, C. Role of 9-Lipoxygenase and α-Dioxygenase Oxylipin Pathways as Modulators of Local and Systemic Defense. Mol. Plant 2012, 5, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Kang, C.; Rustgi, S.; von Wettstein, D.; Reinbothe, C.; Pollmann, S.; Reinbothe, S. Programmed Chloroplast Destruction during Leaf Senescence Involves 13-Lipoxygenase (13-LOX). Proc. Natl. Acad. Sci. USA 2016, 113, 3383–3388. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Vick, B.A.; Farmer, E.E. Dinor-oxo-phytodienoic acid: A new hexadecanoid signal in the jasmonate family. Proc. Natl. Acad. Sci. USA 1997, 94, 10473–10478. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, B. Differential Expression Pattern of an Acidic 9/13-Lipoxygenase in Flower Opening and Senescence and in Leaf Response to Phloem Feeders in the Tea Plant. BMC Plant Biol. 2010, 10, 228. [Google Scholar] [CrossRef]
- Sakharov, I.Y.; Ardila, G.B. Variations of Peroxidase Activity in Cocoa (Theobroma Cacao L.) Beans during Their Ripening, Fermentation and Drying. Food Chem. 1999, 65, 51–54. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Tang, L.; Qiao, F.; Liu, J.; Li, X. Physiological and Transcriptomic Analyses Reveal the Mechanisms Underlying Methyl Jasmonate-Induced Mannitol Stress Resistance in Banana. Plants 2024, 13, 712. https://doi.org/10.3390/plants13050712
Yu J, Tang L, Qiao F, Liu J, Li X. Physiological and Transcriptomic Analyses Reveal the Mechanisms Underlying Methyl Jasmonate-Induced Mannitol Stress Resistance in Banana. Plants. 2024; 13(5):712. https://doi.org/10.3390/plants13050712
Chicago/Turabian StyleYu, Jiaxuan, Lu Tang, Fei Qiao, Juhua Liu, and Xinguo Li. 2024. "Physiological and Transcriptomic Analyses Reveal the Mechanisms Underlying Methyl Jasmonate-Induced Mannitol Stress Resistance in Banana" Plants 13, no. 5: 712. https://doi.org/10.3390/plants13050712
APA StyleYu, J., Tang, L., Qiao, F., Liu, J., & Li, X. (2024). Physiological and Transcriptomic Analyses Reveal the Mechanisms Underlying Methyl Jasmonate-Induced Mannitol Stress Resistance in Banana. Plants, 13(5), 712. https://doi.org/10.3390/plants13050712





