Abstract
The combined morphological features of Stratiotes (Hydrocharitaceae) pollen, observed with light and electron microscopy, make it unique among all angiosperm pollen types and easy to identify. Unfortunately, the plant is (and most likely was) insect-pollinated and produces relatively few pollen grains per flower, contributing to its apparent absence in the paleopalynological record. Here, we present fossil Stratiotes pollen from the Eocene of Germany (Europe) and Kenya (Africa), representing the first reliable pre-Pleistocene pollen records of this genus worldwide and the only fossils of this family discovered so far in Africa. The fossil Stratiotes pollen grains are described and compared to pollen from a single modern species, Stratiotes aloides L. The paleophytogeographic significance and paleoecological aspects of these findings are discussed in relation to the Hydrocharitaceae fossil records and molecular phylogeny, as well as the present-day distribution patterns of its modern genera.
Keywords:
aquatic plant; Eocene; Kenya; Messel; paleoecology; plant dispersal route; pollen morphology; tropical forest 1. Introduction
The origin and evolution of extant angiosperms is an ever-changing saga that has, since the birth of paleobotanical sciences, been studied using the morphology and anatomy of both living and fossil plants in combination with their current and past distribution patterns (e.g., [1,2]). In the last two decades, molecular data have become an additional source of information, not only for establishing genetic and phylogenetic relationships among angiosperms (e.g., [3,4,5]) but also for pinpointing the origin of particular lineages in both time and space. It is now the foundation for divergence time estimates and explicit biogeographic analyses (e.g., [6], and references therein). The Hydrocharitaceae, a fully aquatic family comprising 14 genera and c. 136 species (Supplementary Material S1, Table S1), is one of those families that has recently been studied using various modern approaches in attempts to clarify phylogenetic relationships among the genera and species, their time and place of origin, and subsequent divergence and dispersal patterns (e.g., [7,8,9,10]). Chen et al. [9] (p. 8) concluded that the Hydrocharitaceae had an “Oriental” (i.e., S./S.E. Asian-Chinese) origin and that the ancestor of Stratiotes, representing the earliest diverged lineage of the family, dispersed into Europe during the late Cretaceous and Paleocene. They suggested the genus Stratiotes “had diversified widely in this region adapting to wet swamps in the Late Cretaceous” [9] (p. 8), and although this “seems to be inconsistent” with the fossil record of the clade and its relatives, this discrepancy was explained by a preservation “bias in paleobotany.” Indeed, the family’s fossil record is meager and composed only of leaf, seed, and pollen specimens affiliated with certainty to Stratiotes L. (earliest Eocene to Holocene), Ottelia Pers. (middle Eocene to early Oligocene), Hydrocharis L. (middle Eocene to Pleistocene), Enhalus Rich./Thalassia Banks & Sol, ex K.D.Koenig (middle Eocene), Najas L. (late Eocene to Pleistocene), Hydrilla Rich. (late Eocene), and Vallisneria P. Micheli ex L. (late Eocene to Miocene) (Supplementary Material S1, Table S2). There is currently no fossil evidence of Hydrocharitaceae older than the earliest Eocene, and there is not a single reliable fossil record representing this family from South or Southeast Asia or China, the supposed area of origin ([9] “Oriental area”; Supplementary Material S1, Table S2).
Stratiotes is a monotypic genus today. The single living species, Stratiotes aloides L., is distributed throughout Europe and western Central Asia (see Figure 8 in [11]). The plant is a peculiar dioecious perennial, which is submerged in freshwater over winter and rises to the surface to flower in spring. The roots of S. aloides are simple, up to 180 cm long, and loosely attached to the submerged substrate. The linear, sessile, and rigid leaves are up to 160 cm long, spirotristichous, in a rosette, and with spinous-serrate margins. The male inflorescences are 3–6-flowered, but the females bear mostly single flowers. Male flowers have 5–17 stamens surrounded by 20–30 nectaries; female flowers have six pistils surrounded by 20–30 nectaries [11,12]. Stratiotes aloides is entomophilous and mainly pollinated by Diptera flies [13]. The insects are attracted to the flowers by their large white petals, osmophores emitting a smell similar to that of rotten meat, and nectaries [12,14,15]. The pollen grains of S. aloides are isodiametric and spheroidal in shape. They are inaperturate, 42–87 µm in diameter, and characterized by a reticulate sculpture with echinate suprasculpture (having twisted and furrowed echini), and numerous sculpture elements (nanoclavate and nanogemmate) within the lumina (Supplementary Material S1, Table S3). The sculpture, when observed with scanning electron microscopy (SEM), makes pollen of S. aloides unique within the Hydrocharitaceae (e.g., [16,17]), and in combination with pollen shape, size, and aperture configuration, it can be differentiated from any other angiosperm pollen. The fruit of S. aloides is a berry-like capsule containing up to 24 seeds [11]. Seeds are well represented in the fossil record, extending from the beginning of the Eocene to the present time across Europe and into Western Siberia (Supplementary Material S1, Table S2; part of the “West Palearctic” area of [9]). The seeds are borne in fruits that are forced below the water by developing leaves, then sink to the bottom in a gelatinous mass [11], and consequently are more likely than other plant parts to become fossils. Interestingly, S. aloides often reproduces vegetatively via stolons or turions [18], but only leaves (or parts) and seeds have been recognized or reported as fossils.
Here we report on the first proof of pre-Pleistocene fossil Stratiotes pollen from the early Eocene of Messel, Germany (Figure 1) (i.e., [9] “West Palearctic” area), and the “earliest” late Eocene of Kenya (Figure 2) (i.e., [9] “Afrotropical” area). The German and Kenyan pollen types are compared to those produced by modern Stratiotes aloides. The new finds are discussed within the framework of previous fossil occurrences and related (paleo)environmental data, which are compared with extant Stratiotes, ecology, and climate preferences. A dated phylogeny and biogeographic analysis based on the fossil record of Hydrocharitaceae are presented and discussed with regard to the origins and dispersal routes of different Hydrocharitaceae genera.
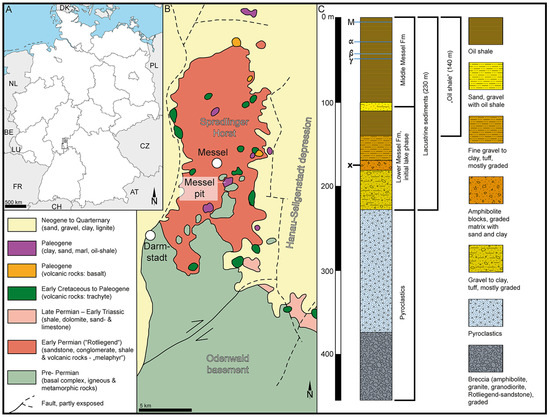
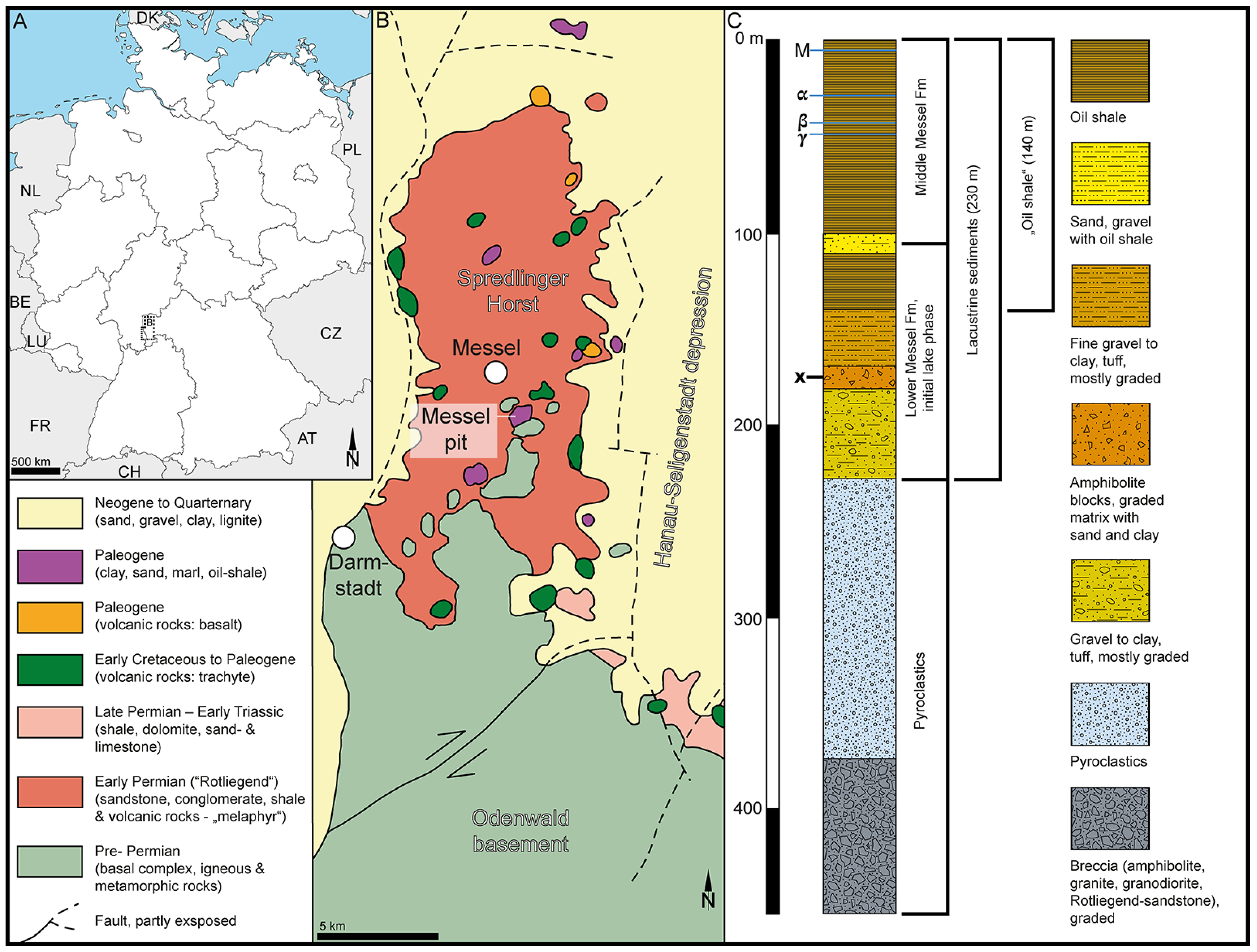
Figure 1.
Geography and geology of the sample site at Messel, Germany, Europe. (A). Schematic map showing the geographic position of Messel pit, Germany, Europe. (B). Simplified geological map of the area surrounding the Messel pit (modified after [19]). (C). Compiled stratigraphic profile (modified after [20]). Stratigraphic level of sample comprising the fossil Stratiotes is marked with an x.
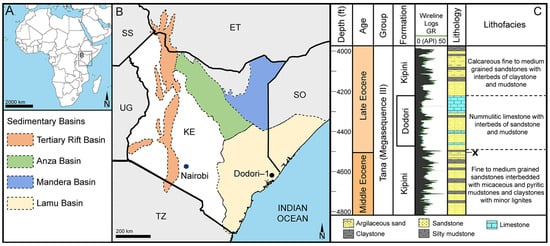
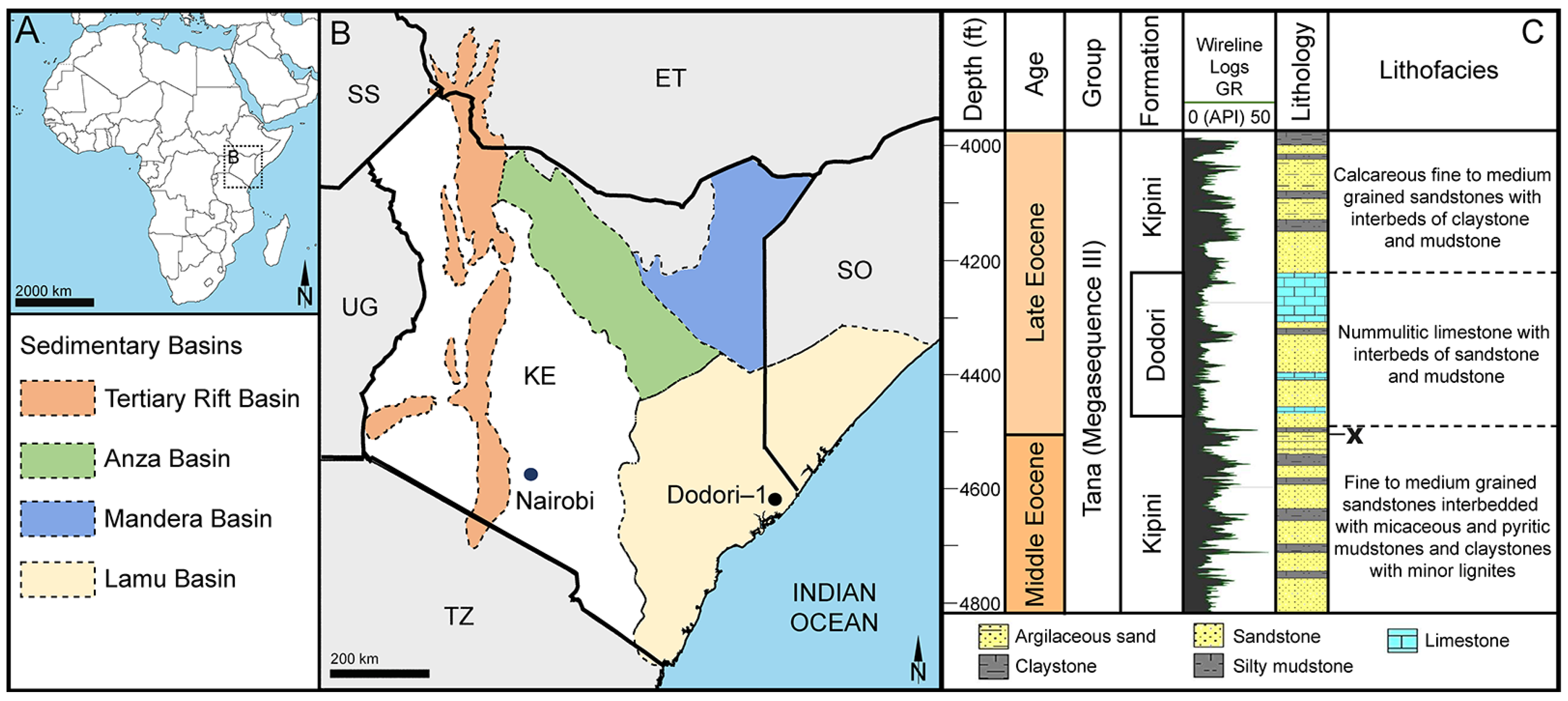
Figure 2.
Geography and geology of the sample site at Dodori, Kenya, Africa. (A,B). Schematic maps showing the geographic position of the Dodori-1 well and major geological formations. (C). Compiled stratigraphic profile (modified after [21]). Stratigraphic level of sample comprising the fossil Stratiotes is marked with an x.
2. Results
2.1. Systematic Description
Order: ALISMATALES R.Br ex Bercht & J.Presl
Family: HYDROCHARITACEAE Juss.
Genus: Stratiotes L.
Species: Stratiotes sp., Messel morphotype (MT), pollen close to Stratiotes aloides (Figure 3; Supplementary Material S1, Table S3).
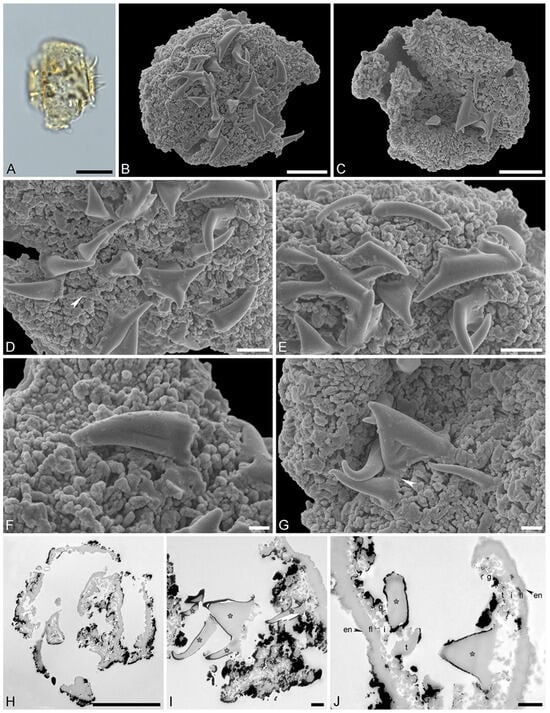
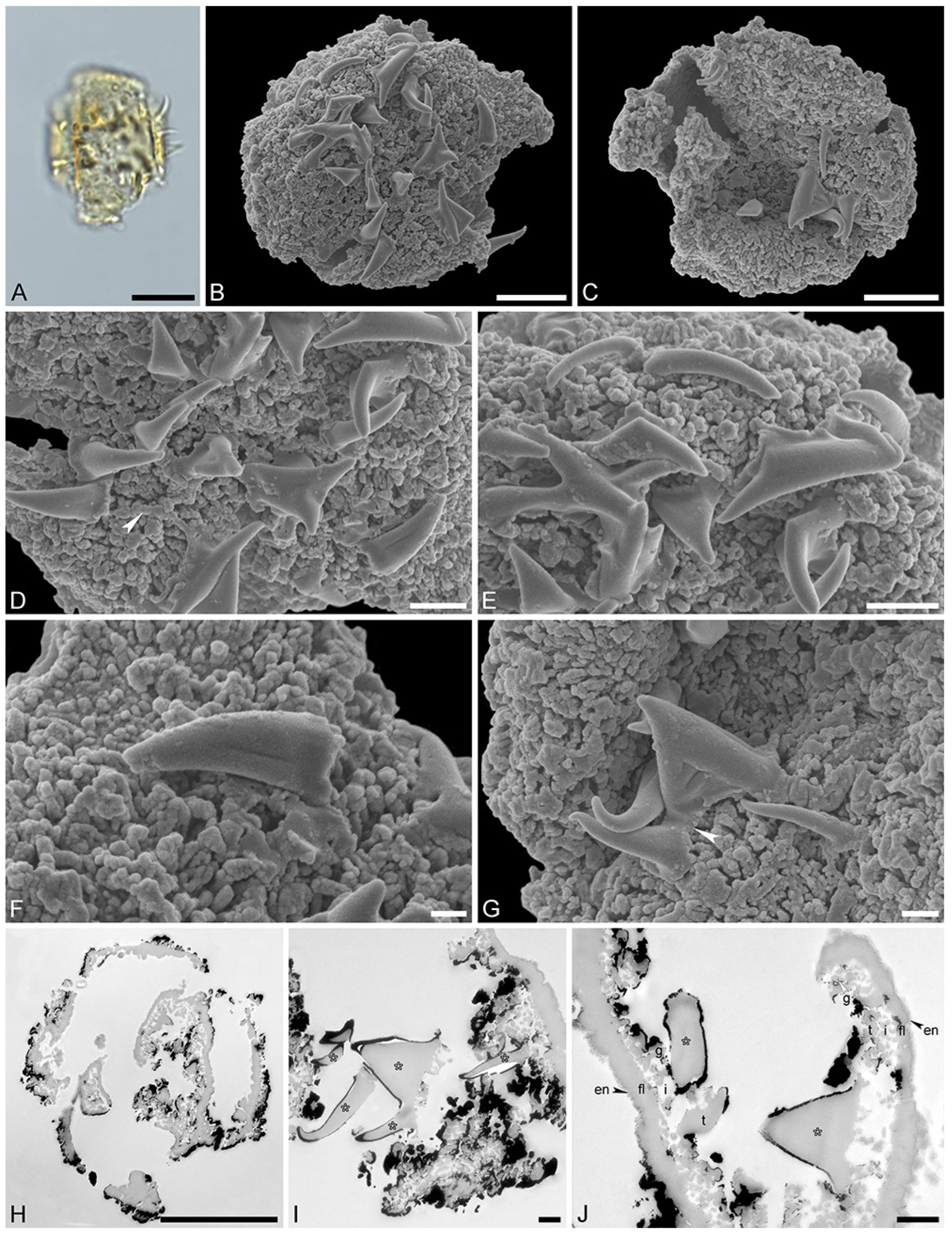
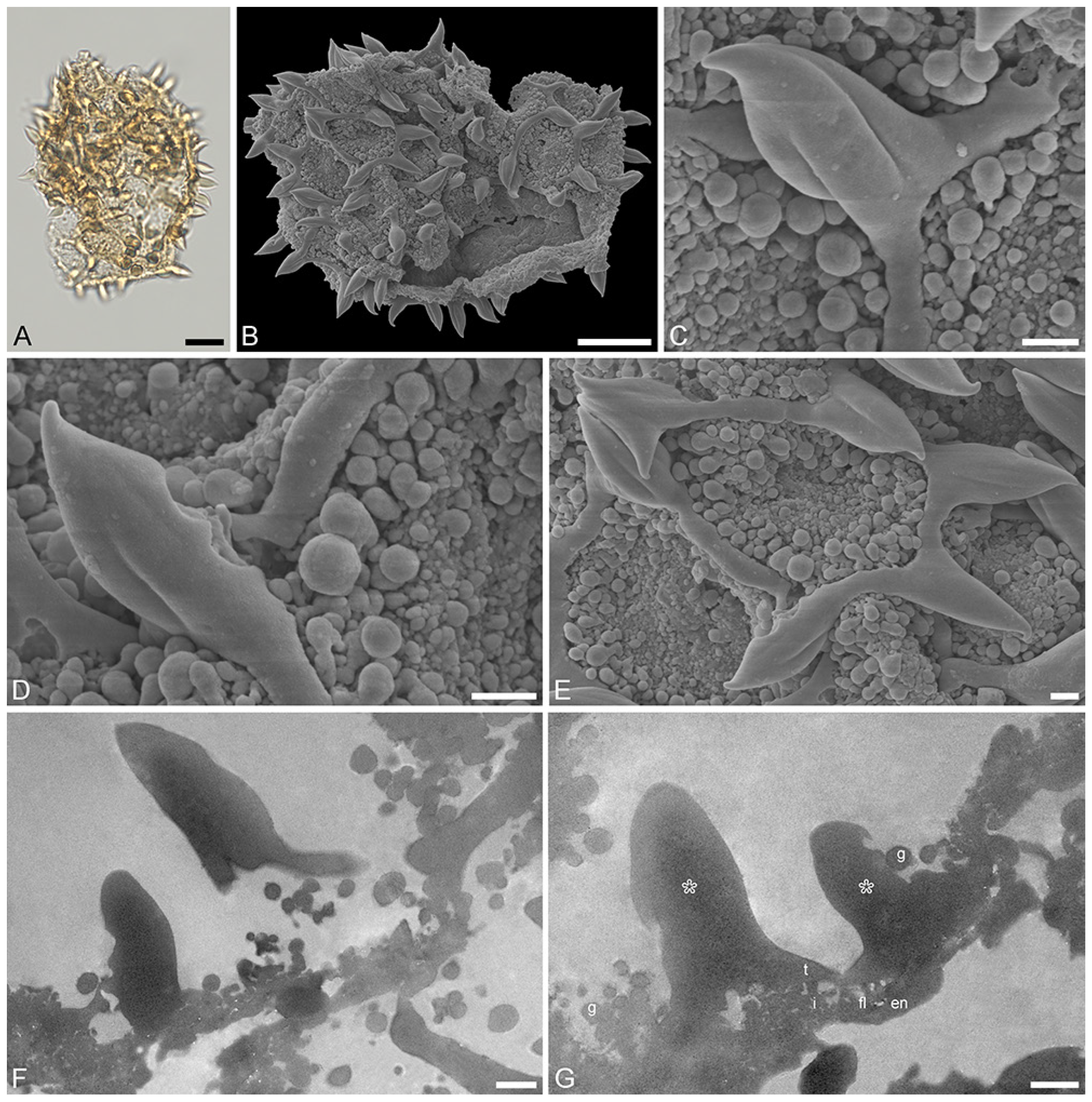
Figure 3.
Fossil Stratiotes pollen from Messel, early Eocene, Germany, Europe. LM (A), SEM (B–G), and TEM (H–J) micrographs. (D,E) Close-up showing reticulum, columellae supporting the eroded murus (white arrowhead). (F,G) Close-up showing echini, murus between echini (white arrowhead). (H) Cross-section of pollen grain, unstained, TEM. (I,J) Detail showing cross-sections of pollen wall with thin compact-continuous endexine (en, black arrowheads), thick, compact-continuous footlayer (fl), alveolate-granular infratectum (i), semitectum (t), supratectal elements (echini, asterisk), and smaller sculpture elements such as granules (g, white arrowhead); note the electron-dense gold layer, unstained, TEM. Scale bars—10 μm (A–C,H), 2 μm (D,E), 1 µm (F,G,I,J).
“Description”: Pollen, monad, P/E ratio isodiametric, outline circular to elliptic; diameter 21–25 μm in LM, 18–23 μm in SEM (Figure 3A–C); inaperturate; exine ca. 1.8 μm thick (excluding echini) in LM; semitectate; sculpture echinate in LM, reticulate with echinate suprasculpture in SEM, and nanogemmate, nanoareolate to nanorugulate inside lumina (SEM; Figure 3D–G); echini 3–3.5 μm in height, 10–12 per 100 µm2 pollen surface, straight to twisted, sometimes fused (Figure 3F,G), with furrows extending from base towards apex (SEM); exine ca. 1.8 µm thick (LM), composed of three layers (transmission electron microscopy [TEM]; Figure 3H–J); thin compact-discontinuous endexine; thick compact-continuous footlayer; alveolate-granular infratectum with varying granulae clusters; discontinuous tectum (semitectate); and supratectal elements echini (TEM; Figure 3H–J).
“Remarks”: The fossil Stratiotes pollen grain (Messel MT) from the early Eocene of Messel, Germany, shares diagnostic characteristics with fossil Stratiotes pollen from the earliest late Eocene of Kenya (Dodori MT; Figure 4 and Figure 5), as well as extant pollen of S. aloides in LM, SEM, and TEM (Figure 6; Supplementary Material S1, Table S3; Supplementary Material S3, Figures S1–S4). Some morphological (SEM) and ultrastructural (TEM) differences between the Messel MT and extant Stratiotes pollen can be observed. First, the Messel MT pollen is eroded; it is infolded and has lost much of the sexine/tectum layer of the pollen wall. Many of the echini, which are usually abundant and seen as supratectal elements on the muri of the reticulum, are missing. Still, remnants of the muri can be seen in Figure 3D,G (arrows). Despite the poor preservation state, the pollen grain can be affiliated with the genus Stratiotes based on, among others, the morphology of the echini, showing the characteristic furrows that run from the base of the echinus towards the apex and the ultrastructure of the pollen wall observed with TEM. The main difference between extant Stratiotes pollen and the Messel MT is in size (Supplementary Material S1, Table S3), with the fossil being much smaller (which could be caused by infolding or missing parts in the fossil pollen). Also, the ultrastructure of the Messel MT pollen shows that the infratectum is denser in the fossil compared to that observed in extant pollen, and the footlayer is continuous-compact in the Messel MT versus discontinuous in extant Stratiotes pollen (compare Figure 3J with Figure 6J).
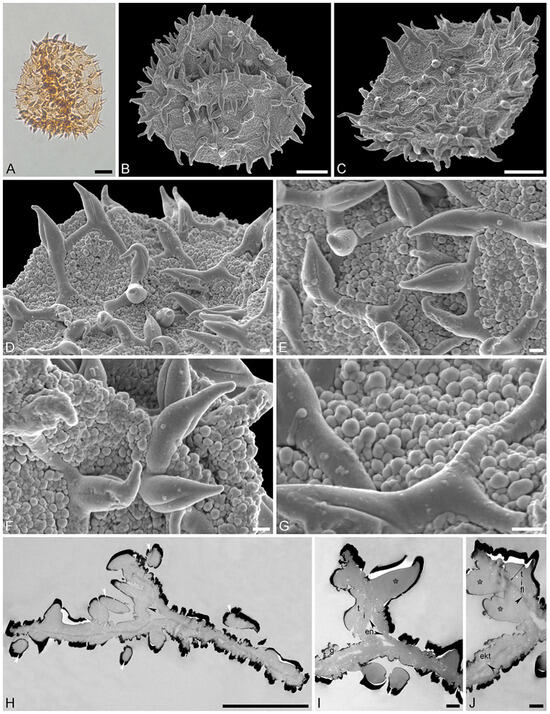
Figure 4.
Fossil Stratiotes pollen from Dodori, earliest late Eocene, southeast Kenya, Africa. LM (A), SEM (B–G), and TEM (H–J) micrographs. (D) Close-up showing reticulum. (E) Close-up showing reticulum and muri of varying length. (F) Close-up showing twisted and furrowed echinus. (G) Close-up showing gemmate sculpture. (H) Cross-section of pollen grain, endexine pinpointed with black arrowhead, cross-sections through echini pinpointed by white arrowheads, potassium permanganate, TEM. (I) Detail showing cross-section of pollen wall with continuous-compact endexine (en, black arrowheads), granular infratectum (i), semitectum (t), and supratectal elements (echini, asterisk), cross-section through echini pinpointed by white arrowhead, unstained, TEM. (J) Detail showing cross-section of pollen wall with endexine (black arrowheads) and ektexine (ekt) with semitectum (t) alveolate-infratectum (i), thin, continuous-compact footlayer (fl), and supratectal elements (echini, asterisk), potassium permanganate, TEM. Scale bars—10 μm (A–C,H), 1 μm (D–G,I,J).
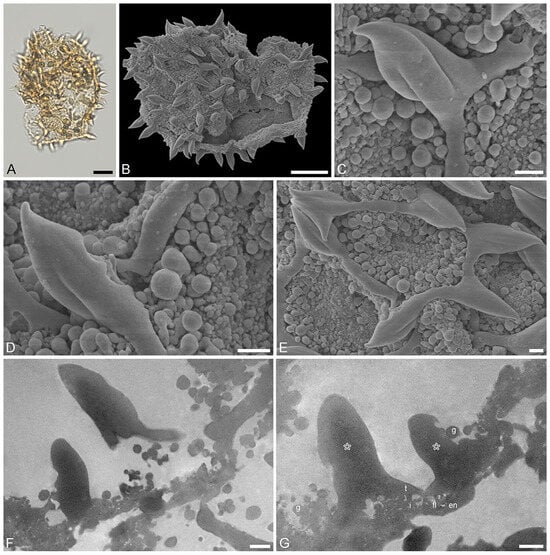
Figure 5.
Fossil Stratiotes pollen from Dodori, earliest late Eocene, southeast Kenya, Africa. LM (A), SEM (B–E), and TEM (F,G) micrographs. (C,D) Close-up showing twisted and furrowed echinus. (E) Close-up showing one brochus of reticulum and muri of varying length. (F,G) Cross-section of pollen wall with compact, more or less continuous endexine (en), thin, compact-continuous footlayer (fl) alveolate-granular infratectum (i), semitectum (t), supratectal elements (echini, asterisk), and smaller sculpture elements such as granules (g), unstained, TEM. Scale bars—10 μm (A–C), 1 μm (D–G).
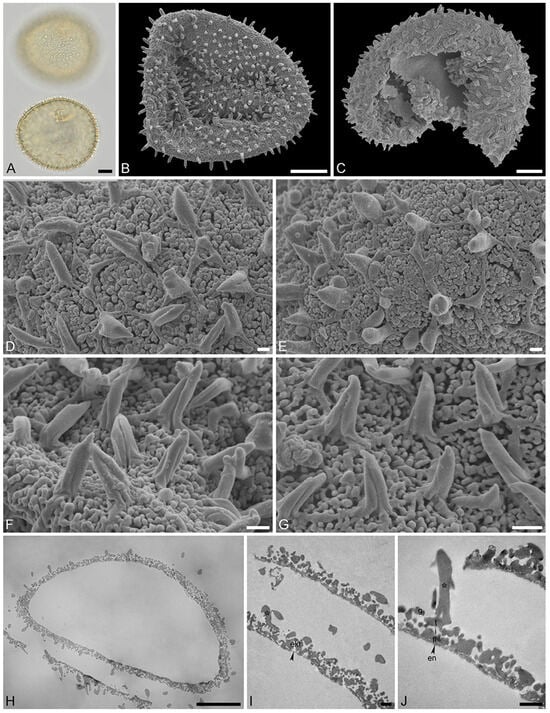
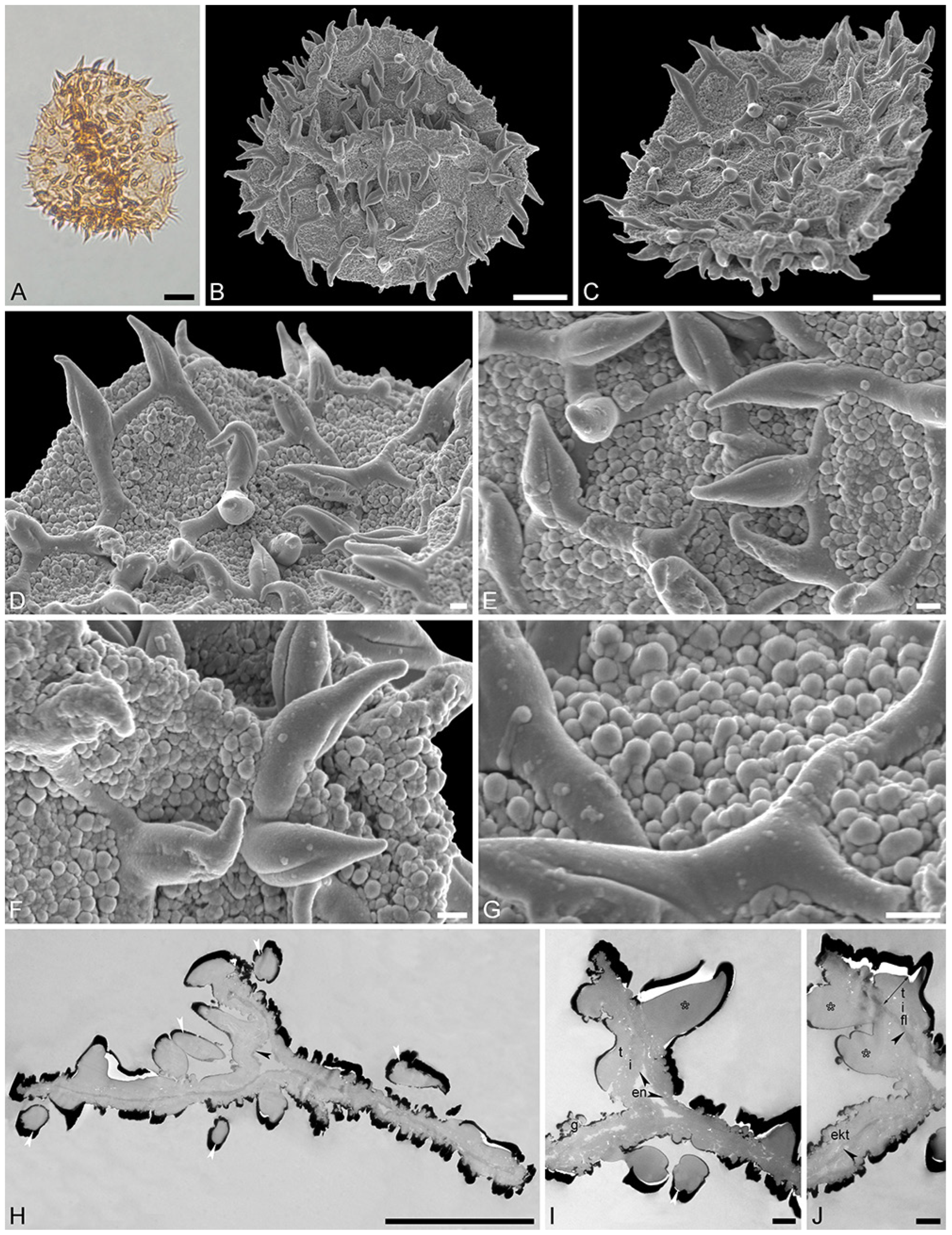
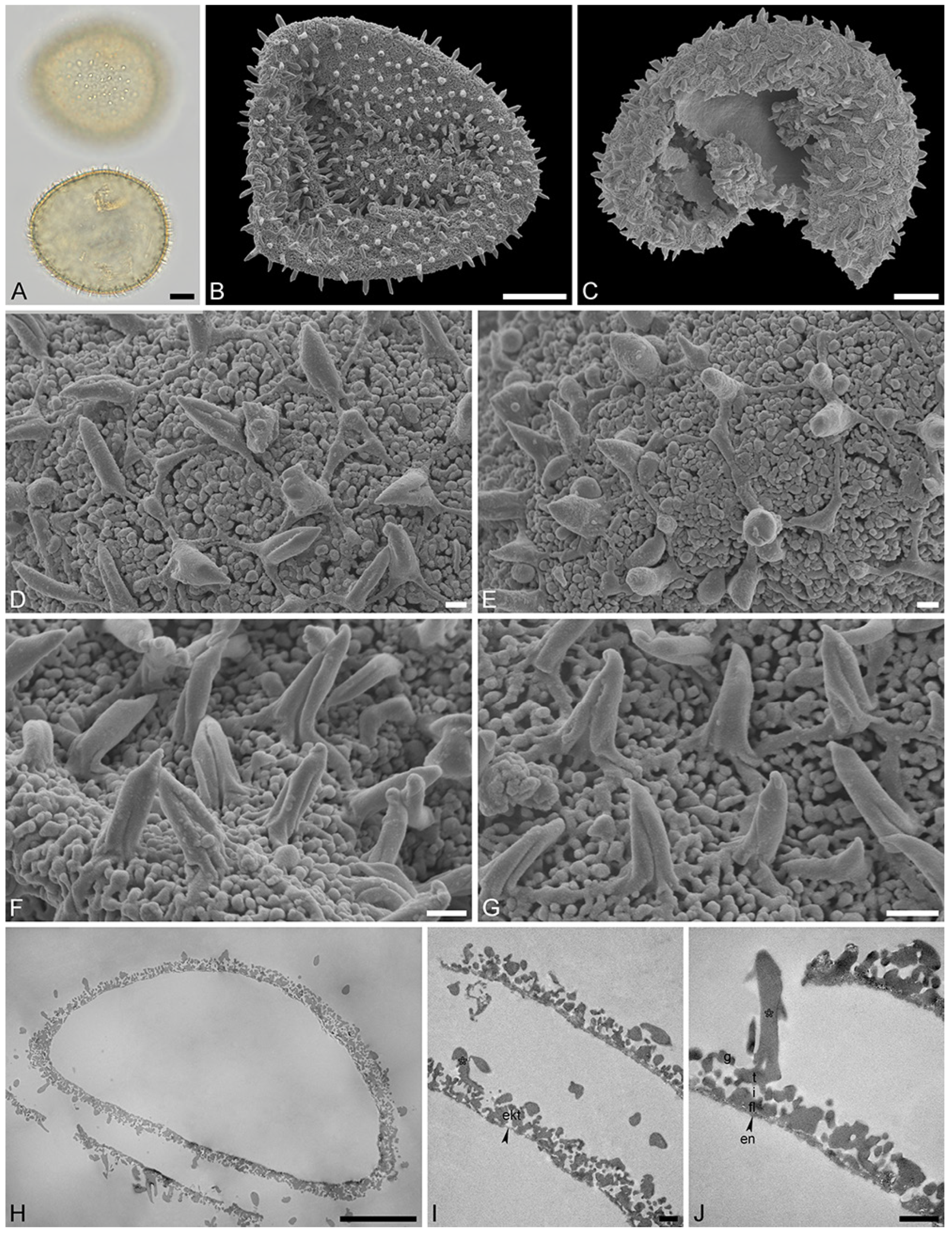
Figure 6.
Recent Stratiotes aloides L. pollen from herbaria specimens (A,B,F,G) from WU0152795; (C–E,H–J) from WU0152793). (A) LM micrographs in upper focus plane and optical cross-section. (B,C) SEM overviews. (D–G) Close-ups of pollen surface showing the reticulum, sculpture elements inside lumina, and twisted, three-furrowed echini atop the muri. (H) Cross-section of a pollen grain in TEM, unstained. (I,J) Detail showing cross-section of pollen walls with thin compact-continuous endexine (en, black arrowhead), compact-discontinuous footlayer (fl), alveolate-granular infratectum (i), semitectum (t), supratectal elements (echini, asterisk), and smaller sculpture elements such as granules (g), unstained, TEM. Scale bars—10 μm (A–C,H), 1 μm (D–G,I,J).
Hydrocharitaceae leaves have been reported from Messel [22]. Surprisingly, the characteristic seeds of Stratiotes have not been recorded among the rich record of fruits and seeds [23]. Also, in a study on dispersed pollen from Messel [24], Thiele-Pfeiffer described a new species, Punctilongisulcites microechinatus Thiele-Pfeiffer, which was assigned to Hydrocharitaceae. The author discussed Stratiotes as one of the few potential source genera. However, she was not able to recognize the characteristic reticulate pattern between the spines and left the generic assignment open. Bouchal et al. [25] have now shown that the Punctilongisulcites microechinatus pollen type of Thiele-Pfeiffer [24] is sulcate and affiliated with Arecaceae.
Species: Stratiotes sp., Dodori MT, pollen close to Stratiotes aloides (Figure 4 and Figure 5; Supplementary Material S1, Table S3).
“Description”: Pollen, monad, P/E ratio isodiametric, shape spheroidal, circular in polar and equatorial views; diameter 48–60 μm in LM, 50–56 μm in SEM (Figure 4A–C and Figure 5A,B); inaperturate; exine 1.7–2.9 μm thick (excluding echini) in LM; semitectate; sculpture reticulate and echinate in LM, reticulate with echinate suprasculpture in SEM, and nanogemmate to microgemmate inside lumina (Figure 4D–E and Figure 5C–E); muri forming ± pentagonal pattern, echini at intersection of muri; muri rounded, sometimes discontinuous (Figure 4D and Figure 5E), 1.4–5.5 μm long, 0.6–1.2 μm wide (SEM); lumina 3.6–9.8 μm in diameter (SEM); echini 4.5–5.5 μm in height, 3–10 per 100 µm2 pollen surface, straight or twisted (Figure 4F and Figure 5C,D), with three furrows extending from the base towards apex (SEM); exine 1.7–2.9 µm thick, composed of three layers (TEM; Figure 4H–J and Figure 5F,G); compact-discontinuous endexine; continuous footlayer; alveolate to granular infratectum with varying granulae clusters; discontinuous tectum (semitectate); and supratectal elements echini (TEM).
“Remarks”: The fossil Stratiotes pollen (Dodori MT) from the earliest late Eocene of Kenya, Africa, shares diagnostic characteristics with extant pollen of S. aloides in LM, SEM, and TEM (Figure S4; Supplementary Material S1, Table S3; Supplementary Material S3, Figure S1–S4). Some morphological (SEM) and ultrastructural (TEM) differences between the fossil and extant pollen can be observed. The sculpture elements of S. aloides pollen are shorter and narrower but more frequent than those of the Dodori MT (SEM). The muri are usually longer and broader in the Dodori MT, and the lumina are generally larger (SEM). The echini are taller in the Dodori MT and fewer in number per 100 μm2 pollen surface. Also, sculpture elements inside lumina are nanogemmate to microgemmate, but nanoclavate to nanogemmate in pollen of S. aloides (SEM) (Supplementary Material S1, Table S3). The ultrastructure of the Dodori MT pollen wall is only slightly different from that of extant S. aloides, mainly in terms of thickness. The infratectum of the Dodori MT is more compact than that of the S. aloides, the supratectal elements (echini) in the fossil are also larger than in the modern pollen, and the footlayer is continuous-compact in the Dodori MT versus discontinuous in extant Stratiotes pollen (compare Figure 4J with Figure 6J).
We are not aware of any previous Hydrocharitaceae (Stratiotes) fossil records from this locality or of any other Cenozoic plant-bearing sediments from Africa.
2.2. Phylogeny of Hydrocharitaceae
Our IQTREE and MrBayes analysis of the molecular data from Bernardini and Lucchese [10] retrieved a strongly supported topology coherent with this previous study (Supplementary Material S4). All genera are retrieved as monophyletic with good support. Two major clades are retrieved with strong support: a clade including Lagarosiphon Harv. as sister to Elodea Michx sensu lato and Ottelia plus Blyxa Noronha ex Thouars (clade B) (100/100/1 Bootstrap/aLRT/Posterior Probability), and a clade including Limnobium Rich. plus Hydrocaris as sister to a clade of Najas plus a clade of the seagrasses (Halophila Thouars, Enhalus, and Thalassia) plus a clade of Hydrilla as sister to Nechamandra Planch. plus Vallisneria (clade A) (82.5/83/1). The placement of Stratiotes as sister to the rest of the Hydrocharitaceae was supported, albeit weakly, in IQTREE (75.3/80), but not in the Bayesian analysis.
2.3. Dated Phylogeny
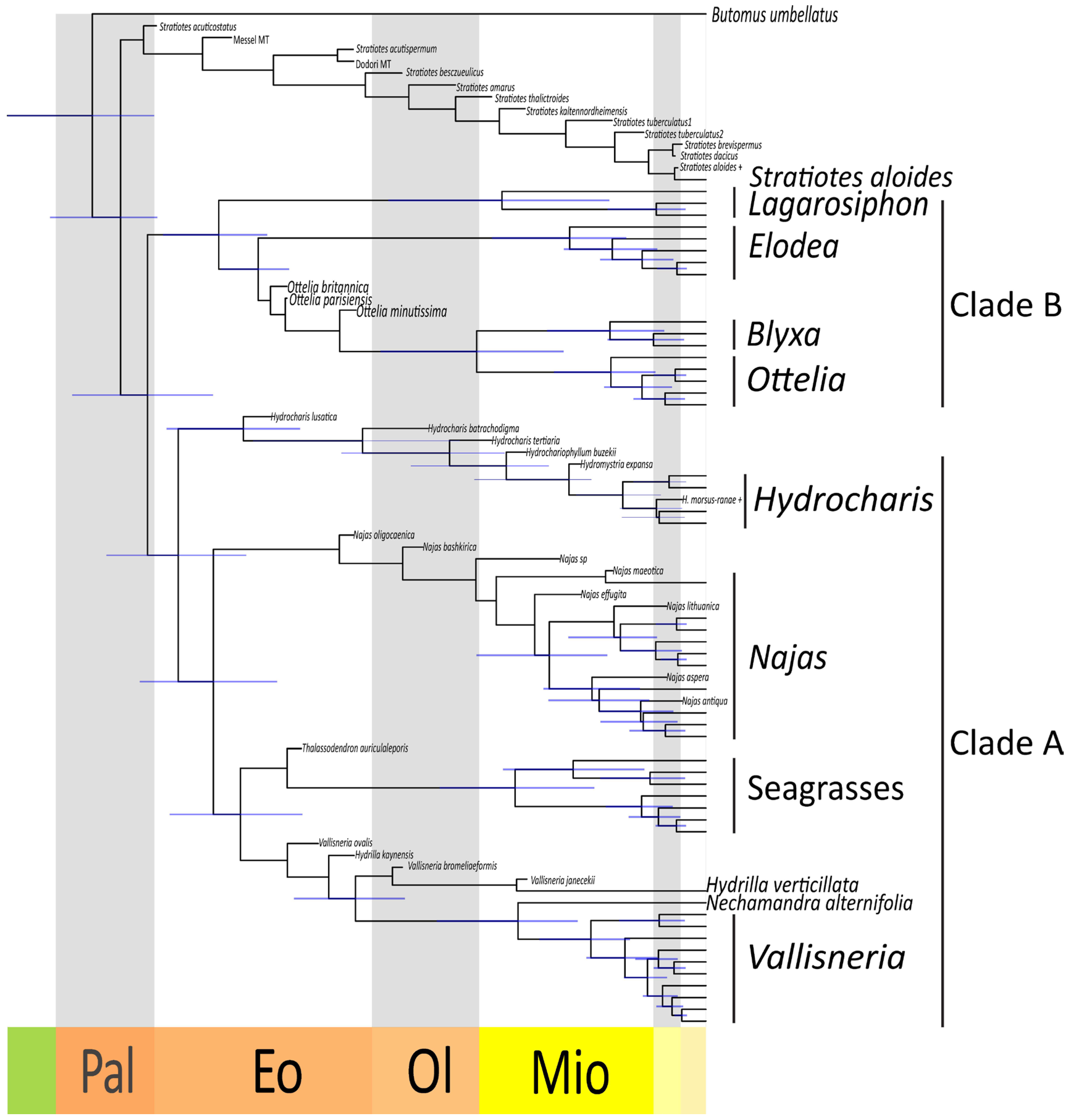
Our dated phylogeny inferred the origin of crown-group Hydrocharitaceae between the late Cretaceous and the Paleogene (67–56 Ma) (Figure 7). The divergence of Stratiotes from the rest of the family is estimated to have happened between 64 and 50 Ma. Clade A appears to be slightly younger than clade B, the former having originated between 55 and 45 Ma and the latter having originated between 61 and 47 Ma. The genus Hydrilla diverged rather early from the Nechamandra-Vallisneria clade (42–31 Ma). Among the crown groups of the extant genera, the oldest is Najas, which originated between 28 and 14 Ma. Of similar age are the Ottelia-Blyxa clade (33–14 Ma), the Nechamandra-Vallisneria clade (27–13 Ma), the Seagrass clade (27–11 Ma), and the genus Lagarosiphon (32–10 Ma). The genus Elodea sensu lato originated between 22 and 8 Ma, and the genus Vallisneria between 17 and 8 Ma. Younger are the crown groups of the genus Ottelia (15–5 Ma), the genus Blyxa (16–4 Ma), and the genus Hydrocharis sensu lato (13–5 Ma).
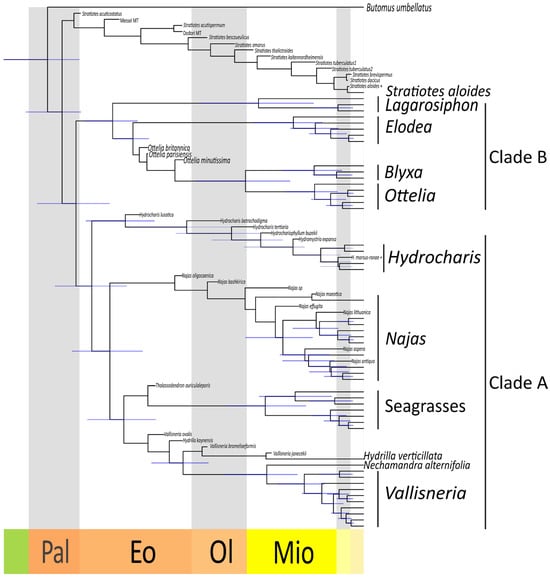
Figure 7.
Dated phylogeny of the Hydrocharitaceae. Maximum Clade Credibility tree obtained after removing the fossil taxa from the posterior trees. In addition, 95% Highest Posterior Density intervals are shown at nodes (blue lines). Late Cretaceous (green), Paleocene (Pal), Eocence (Eo), Oligocene (Ol), Miocene (Mio), Pliocene (light yellow), Pleistocene (beige).
2.4. Biogeographic Analysis
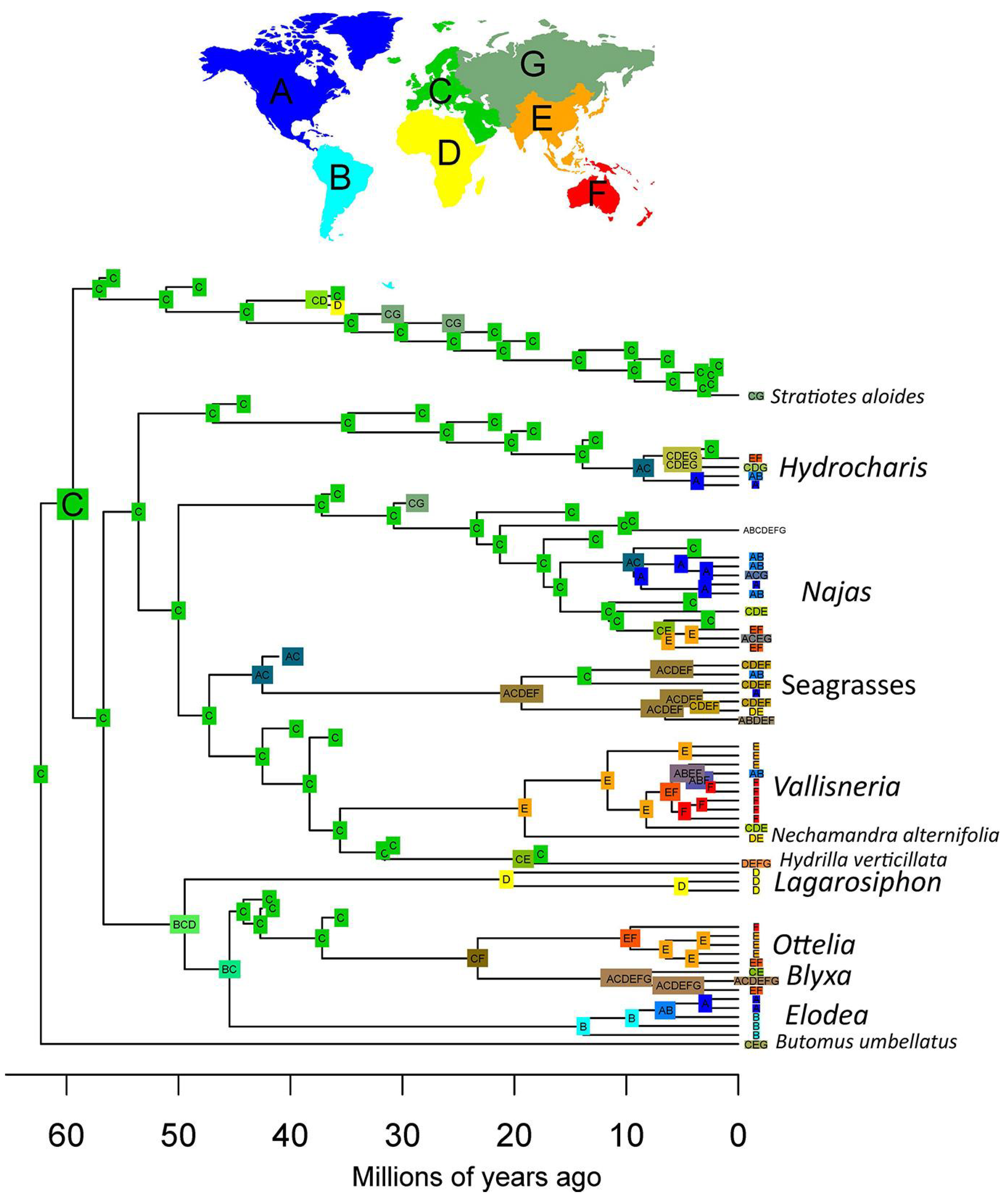
The results of our biogeographical analysis show that most of the ancestral nodes of Hydrocharitaceae were distributed in Europe (Figure 8). The crown node of clade A is inferred to have had a wider distribution including Europe, Africa, and South America, with extirpation leading to an African origin of Lagarosiphon, a South American origin of Elodea sensu lato, and a European origin of the stem of the Ottelia-Blyxa clade. This latter group has a much more complex later history, indicating a widespread distribution of Blyxa and an Australian–Southeastern origin of Ottelia. Representatives of clade B were distributed in Europe for most of their evolutionary history, with the exception of the seagrasses, which appear to have obtained a widespread distribution early in their history. The current rather widespread distribution of Hydrocharis and Najas appears to have been the result of recent dispersals outside Europe (i.e., through North America or through Southeast Asia), while Vallisneria shows an ancestral distribution in Southeast Asia with later dispersal to the Americas.
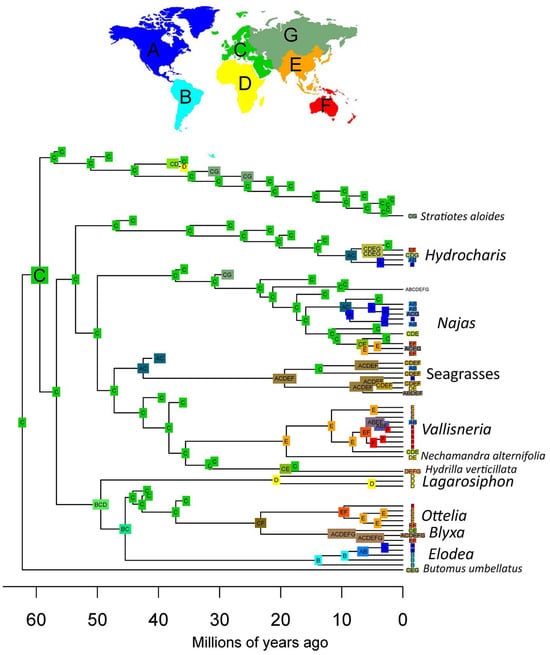
Figure 8.
Biogeographical reconstruction of the Hydrocharitaceae. Reconstruction produced using the Dispersal–Extinction–Cladogenesis (DEC) model, scoring absences in fossil tips as uncertain. The reconstruction shows a European origin of most extant clades.
2.5. Climate Preferences Hydrocharitaceae
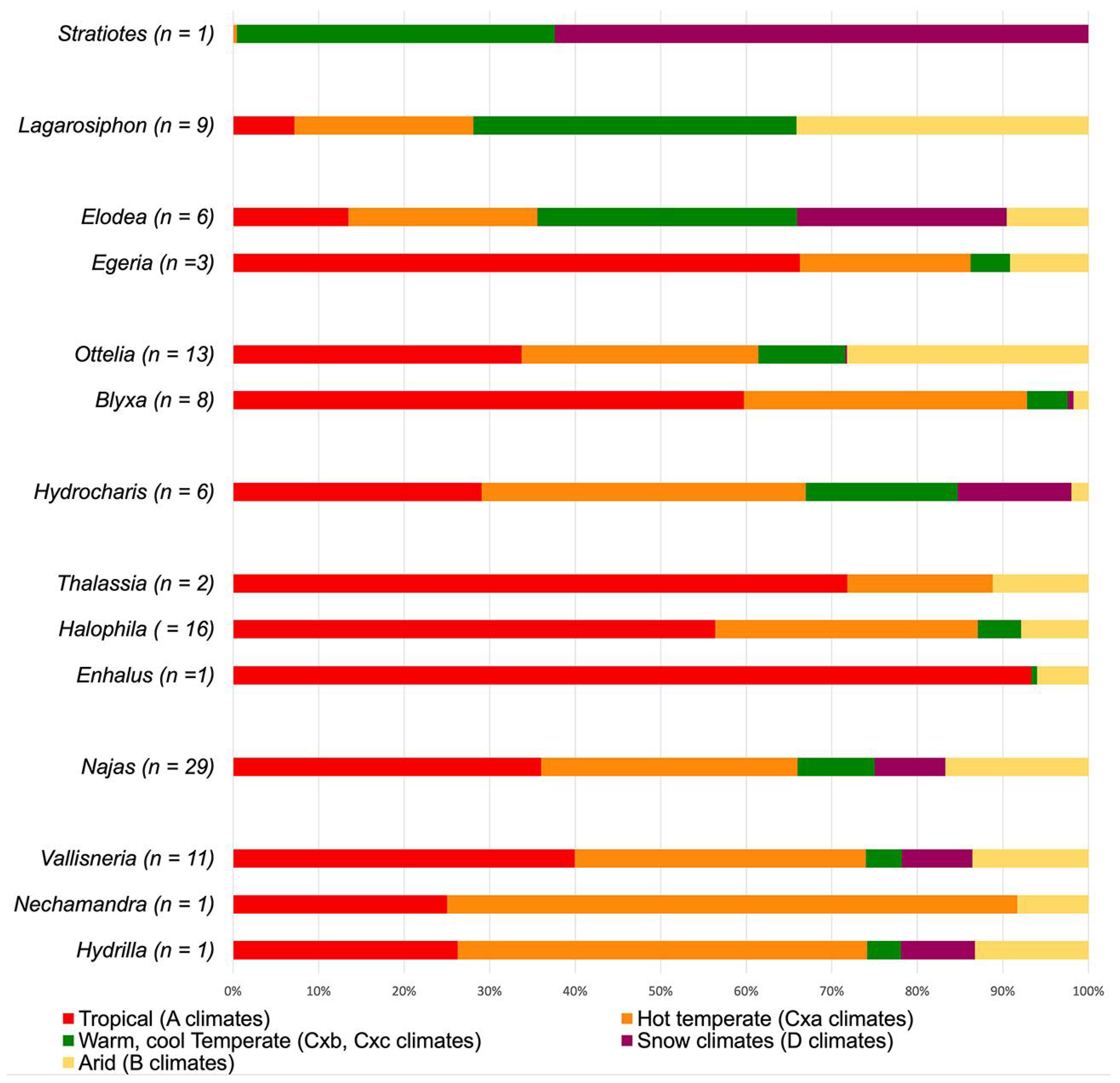
To estimate the paleoclimatic preferences of Stratiotes and other Hyrdocharitaceae, we compiled geographic occurrences and climatic preferences (in total 37.498 data sets, Supplementary Material S1, Table S5) of 107 species belonging to 13 out of the 14 extant Hyrdocharitaceae genera listed in POWO [26]. We used GBIF and prevailing climate across species distribution areas ([27], Supplementary Material S2). For practical reasons, the climatic aspects of Hydrocharitaceae are here discussed by genera and clades following the phylogenetic framework of Chen et al. [9]. Since Hydrocharitaceae grow submerged in waterbodies, precipitation is not a restricting factor. Therefore, we omitted the second letter of the Köppen climate types for this family.
Stratiotes is the first diverging genus in the family’s phylogenetic tree. It is monotypic and presently restricted to western Eurasia, where it thrives mainly under temperate and snow climates, with warm (Cfb, Dfb) or cool to short summers (Dfc) (Figure 9). In the Po valley, Italy, the northern coast of the Black Sea, and north of the Caspian Sea, between Dnepr and Wolga, this species extends into humid temperate and snow climates with hot summers (Cfa, Dfa). Within Hydrocharitaceae, Stratiotes is today the only genus confined to temperate climates.
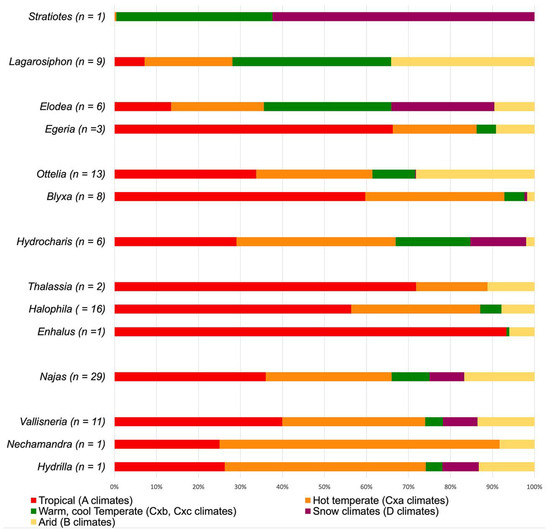
Figure 9.
Main climatic profiles of extant Hydrocharitaceae. Climatic parameters are defined in Supplementary Material S2. Graphs/bar charts including all Köppen climate types are available in Supplementary Material S2. n = number of investigated species.
Lagorosiphon and Appertiella C.D.K.Cook et L.Triest belong to the first diverging group in Clade B and show a Madagascan and African distribution. Here, Lagorosiphon occupies fully tropical climates (Aw), a variety of hot and cold arid steppe and desert climates (B-climates), and temperate climates with hot and warm summers (C-climates) (Figure 9). No occurrence data was available for Appertiella. The second group in Clade B includes Egeria Planch. and Elodea [9]. Egeria is restricted to South America and thrives under tropical (mainly Aw; extending into Af, Am) and arid desert and steppe climates with hot summers (mainly BSh; extending into BWh) but is also found in temperate climates with hot to warm summers (mainly Cfa; extending into Cfb, Cwa, Cwb). Elodea is native to North and South America, but absent in Central America. In the Americas, this genus shows the widest climatic range, present in fully tropical (Af, Am, Aw) and in hot and arid desert and steppe climates (B-climates). It is mainly present in temperate and snow climates where summers are either hot or warm (various C- and D-climates). Some species [Elodea bifoliata H.St.John, E. canadensis Michx., E. nutalli (Planch.) H.St.John] extends this range into snow climates with cold and short summers (Dfc, Dsc) (Figure 9). The last group in Clade B consists of Blyxa and Ottelia. The geographic distribution of both genera includes Africa, India, East Asia, and most of Australasia, with only Ottelia extending into South America. Blyxa is present in tropical (Af, Am, Aw) and temperate climates with hot to warm summers (Cfa, Cfb, Cwa, Cwb). Only some species extend into arid [BSh, BWh; Blyxa auberti Rich., B. echinosperma (C.B.Clarke) Hook.f., B. octandra (Roxb.) Planch. ex Thwaites] and snow climates [Dwa, Dwb; B. auberti, B. japonica (Miq.) Maxim, ex Asch. et Gürke]. Ottelia grows in tropical (mainly Aw, extending into Af, Am), arid climates (mainly BSh; extending into BSk, BWh, BWk) as well as temperate climates with hot or warm summers (mainly Cfa, Cfb, Cwa, Cwb; extending into Csa, Csb). Only Ottelia alismoides (L.) Pers. occurs in snow climates with hot or warm summers (Dfa, Dfb) (Figure 9).
The first diverging group in Clade A comprises Hydrocharis (incl. Limnobium), which occurs on all continents except Antarctica. Its climatic preferences range from fully tropical (mainly Aw, Am; extending into Af) to temperate and snow climates with hot to warm summers (mainly C-climates, extending into D-climates) and occasionally into snow climates with cold and short summers (Dfc, Dwc). Only Hydrocharis morus-ranae L. is widespread in the Dfc climate of the Eurasian Taiga (Figure 9). The second diverging group in Clade A includes all marine members of Hydrocharitaceae (Thalassia, Halophila, and Enhalus). All taxa in this group are mainly found between the Tropic of Cancer and the Tropic of Capricorn. There, they prevail in fully tropical (Af, Aw, Am), arid (mainly BSh, BWh; extending into BSk, BWk), and temperate climates (mainly Cfa, Csa). Najas forms the third group diverging in Clade A, showing a nearly global distribution with a wide climatic tolerance (various A-, B-, and C-climates). Some species [Najas flexis (Willd.) Rostk. Et W.LE.Schmidt, N. gracillima (A.Braun ex Engelm.) Magnus., N. graminea Delile, N. guadalupensis (Spreng.) Magnus, N. marina L., and N. tenuissima (A.Braun ex Magnus) Magnus] thrive well under snow climates with hot to cool short summers (mainly Dfa, Dfb, Dfc; extending into Dsc, Dwa, Dwb, Dwc) (Figure 9). The fourth and last group in Clade A comprises Vallisneria, Nechamandra, and Hydrilla. Hydrilla verticillata (L.f.) Royle, the single species of this genus, is native to Eurasia, Australasia, and tropical Africa. Its wide climatic amplitude includes fully tropical (Af, Am, Aw), arid desert and steppe (mainly BSh, BSk; extending into BWk), and temperate climates with hot to warm summers (mainly Cfa, Cfb, Cwa). In Central and East Asia, this species thrives under snow climates with hot to cool short summers (mainly Dfb). Nechamandra is restricted to Southeast Asia and tropical (Aw) and temperate climates with hot summers (Cfa, Cwa), occasionally extending into hot desert climates (BWh). The 16 Vallisneria species are widely distributed on most continents but rare in South America (only found in Colombia). Some species’ climatic preferences (Vallisneria annua S.W.L.Jacobs et K.A.Frank, V. caulescense F.M.Bailey et F.Muell., V. erecta S.W.L.Jacobs, V. rubra, (Rendle) Les et S.W.L.Jacobs, V. triptera S.W.L.Jacobs et K.A.Frank) include tropical (mainly Aw; extending into Af, Am) and arid desert and steppe climates (mainly BSh, BWh; extending into BSk, BWk). Other members of this genus [V. denseserrulata (Makino) Makino, V. natans (Lour.) H.Hara, and V. spinulosa S.Z.Yan] thrive predominantly in temperate and snow climates with hot to warm summers (mainly Cfa, Cfb, Dfa, Dfb). Vallisneria americana Michx., V. nana R.Br., and V. spiralis L. are more generalistic in their climatic preference (occurring under various A-, B-, C, and D-climates) (Figure 9).
Based on the climatic preference of Hydrocharitaceae, it becomes apparent that most species prefer tropical to warm temperate climates (Figure 9). The outlier of this pattern is the monotypic Stratiotes, which is restricted to warm temperate and snow climates of western Eurasia.
3. Discussion
3.1. The Paleophytogeographic History of Stratiotes
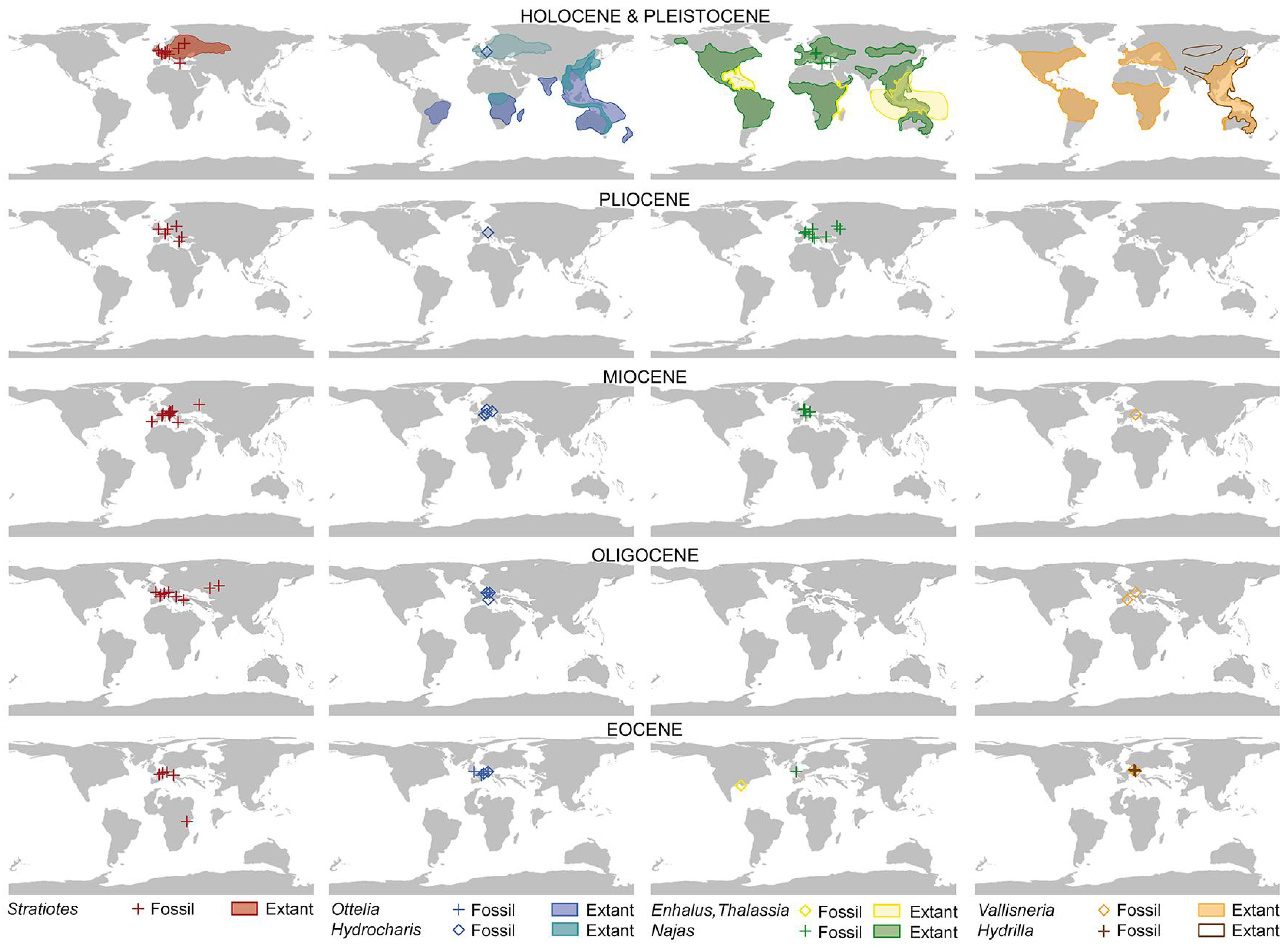
The oldest Stratiotes record reported so far is from the earliest Eocene of the UK (~56 Ma; Supplementary Material S1, Tables S2 and S6) (Figure 10). The timing and provenance of the known fossil record suggest a West European origin for Stratiotes, followed by an eastward dispersal across Europe into northwestern Asia during the Eocene. The genus had a European west–east transcontinental distribution by the latest Eocene and extended into European Russia and Western Siberia from the Oligocene onwards. Apparently, the taxon then thrived in various parts of Europe and Western Siberia until the Pliocene/Pleistocene (Supplementary Material S1, Table S2). Recent phylogenetic studies ([9] Figure 1; [10] Figure 1) place Stratiotes as the earliest diverging lineage and sister to the remaining Hydrocharitaceae. If the family originated in South/Southeast Asia as inferred by Chen et al. [9], it would have needed to radiate quickly into Europe where it thrived for the next 50+ Ma, while going extinct in its area of origin. The early Eocene fossil records and phylogenetic position of Stratiotes would point towards Europe as the focal point of origin for the genus/family. This is consistent with our new finds, one of which represents an Eocene lineage of Stratiotes in sub-Saharan Africa, an area that today hosts the second diverging lineage of the family, Lagarosiphon. The fossil record of Stratiotes has until now been restricted to Europe and Western Siberia (Figure 10) and composed mostly of entire seeds or single valves of germinated seeds. The lack of Stratiotes in the fossil plant record of Africa is not surprising. Stratiotes fossils have until now been confined to Cenozoic fruit/seed records (Supplementary Material S1, Table S2) and such assemblages are rare in Africa (summarized in [28], Table 5.1 and Figure 5.1). Four fruit/seed floras from the Paleocene to Eocene have been reported in Egypt, along with a single Eocene fruit/seed flora in Sierra Leone [28]. None of the reports on these five floras documents the presence of Stratiotes. The notable absence of dispersed Stratiotes pollen in the pre-Pleistocene global paleopalynological records to date can be attributed to the entomophilous nature of the plants [13]. Stratiotes produce only a small amount of pollen (even none at all) per fertile stamen [15], and extant Stratiotes also reproduce vegetatively [29]. Also, because of the structure and function of the pollen wall (see before), the grains disintegrate easily. Furthermore, the majority of paleopalynological studies rely only on LM, which is not sufficient to determine the generic affinity of this pollen type and can easily lead to misidentification as echinate and the much more common Arecaceae pollen. Eocene palynofloras from Europe, for example, are known for their rich and diverse palm pollen components (e.g., [30]).
Based on the current fossil record (Figure 10), including our new finds, the sole surviving Stratiotes species is a relict of the first phase of Hydrocharitaceae radiation involving intercontinental dispersal towards the Americas, Asia, and Africa from Europe. The inferred late Cretaceous and Paleocene East Asian (Oriental) origin for Hydrocharitaceae/Stratiotes of Chen et al. [9] is not justified by the “fact” that “the genetic diversity centre of the family is in topical Asia” [9] (p. 7), but biased by the according modern-day underrepresentation of the earliest diverging non-Asian lineages. In addition, Chen et al. [9] relied on two (outdated) methods for biogeographic analysis that ignored the phylogenetic distances along their tree. Most of the tips are scored as combined areas (coded as polymorphisms; [9] Figure 2), including the “Oriental area” I, hence, the “Oriental” origin of the family, although only four species scattered across the tree and with different biogeographic affinities (South American, Australian, and sub-Saharan African sisters) have been coded as exclusively E. In the case of Stratiotes (aloides), the “Oriental” area was erroneously included: the species stretches from Europe into Siberia (cf. [11] Figure 8), i.e., covers the “West…” and “East Palearctic” area. Thus, their analysis set-up was strongly biased towards an “Oriental” origin. Repeated intercontinental dispersal is likely in the case of aquatic plants such as the Hydrocharitaceae that are dispersed mainly by birds [31], and in the case of Stratiotes, through interconnected bodies of water via turions that serve as vegetative dispersal units [18]. In addition, the extant distribution of many plant lineages only reflects the latest Cenozoic dispersal and East Asian refugia (e.g., [9,32]).
Based on the available fossil record (Figure 10), niche preferences, and paleogeography, we suggest that Stratiotes originated in the British Islands region in the late Paleocene/earliest Eocene and then dispersed eastwards across Europe and southwards into Africa throughout the Eocene. With tropical and subtropical climate equivalents and corresponding vegetation extending from the equator to mid and high latitudes in Europe, temperature would not have been a barrier to a southward dispersal of Stratiotes from Europe into Africa. Such a ‘northern route’ from Eurasia into Africa during the Eocene was recently proposed for the Picrodendraceae, believed to have dispersed into Africa from the Americas via Europe [33], and the Loranthaceae, believed to have entered Africa from Asia around the same time [34,35]. Paleocene to Eocene palynological studies from northern Africa also indicate the presence of typically Northern Hemisphere taxa, including a group of triporate forms likely related to Betulaceae, Hamamelidaceae, and Juglandaceae, as well as fruits of Fagaceae from Egypt (e.g., [28]). Eocene high sea levels resulted in the widespread availability of lacustrine, lagoonal, and estuarine environments between Europe and Africa during the latest Paleocene until at least the middle-to-late Eocene [36]. These environments would have facilitated southward migration by Stratiotes, even though North Africa was covered by shallow seas with some scattered islands at that time, thus requiring some long-distance dispersal. Interestingly, the vertebrate paleontological record also documents several groups that migrated into Africa from Eurasia (primarily Europe) during the Paleogene, and as Africa was depauperate in vertebrate diversity following the breakup of Gondwana in the late Cretaceous, northern immigrants became established, evolving into several characteristic endemic clades [37]. Why and how Stratiotes was extirpated in Africa can only be hypothesized. On the basis of its co-occurrence with pollen of the mangrove palm, Nypa (F. Grímsson, pers. obs.), a relatively expanded shallow coastal zone and large interior basins were present in northern tropical Africa during the latest Paleocene through the middle Eocene, and a major global decline in mangrove communities in the later Eocene, and at the Eocene–Oligocene transition [36], it seems reasonable to hypothesize the loss of Stratiotes from Africa at the same time. Based on the fossil record, the phylogeny, and the current distribution of modern genera, it is possible that African Stratiotes evolved into Lagarosiphon (?Appertiella), the first diverging genus of sister clade B. Both Lagarosiphon and Appertiella are currently endemic to Africa and/or Madagascar. This scenario would imply that the Stratiotes pollen is primitive within the family.
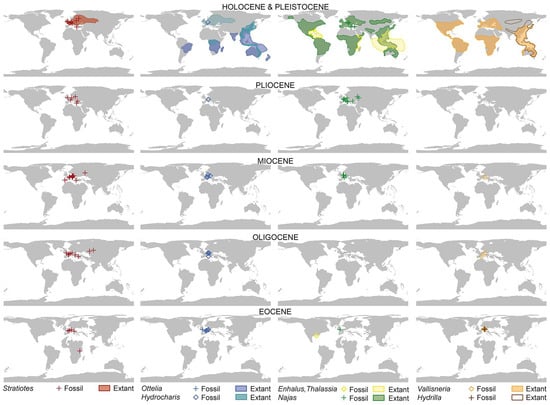
Figure 10.
Distribution of Hydrocharitaceae in time and space. Extant distribution of genera is based on a cleaned georeferenced GBIF dataset (Supplementary Material S1, Table S5) and plotted on the Pleistocene map. Fossil records and ages of paleofloras are based on [22,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155] (for details see Supplementary Material S1, Tables S2 and S6). Palaeomaps modified after [156], epoch maps are based on GeTech palaeo-plate rotational models for the middle of each epoch’s first stage. For simplicity, all fossil occurrences of a genus within an epoch, regardless of their particular stratigraphic range, were plotted on the epoch’s middle first-stage map.
Figure 10.
Distribution of Hydrocharitaceae in time and space. Extant distribution of genera is based on a cleaned georeferenced GBIF dataset (Supplementary Material S1, Table S5) and plotted on the Pleistocene map. Fossil records and ages of paleofloras are based on [22,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155] (for details see Supplementary Material S1, Tables S2 and S6). Palaeomaps modified after [156], epoch maps are based on GeTech palaeo-plate rotational models for the middle of each epoch’s first stage. For simplicity, all fossil occurrences of a genus within an epoch, regardless of their particular stratigraphic range, were plotted on the epoch’s middle first-stage map.
3.2. Ecological and Climatic Preferences of Extant and Fossil Stratiotes
Today, Stratiotes grows in freshwater lakes, sheltered bays, backwaters of rivers, ponds, ditches, and other water bodies that are 2–5 m deep with a stable water level, and is never found in temporary nor greatly fluctuating waters, or in shallow or swiftly flowing water bodies. The plant prefers nutrient-rich muddy substrates and usually occurs in calcareous mesotrophic water [11]. During the latest Paleocene to Eocene, at the time when Stratiotes originated and diverged, all of Europe (excluding the Scandinavian craton and Western Russia) was composed of numerous archipelagoes extending from the British Islands to what today is Asia Minor and Central Asia (e.g., [157,158] and references therein). Numerous embayments and brackish and freshwater lakes occurred across Europe during that time (e.g., [159]), as was true of tropical Africa as well [36], providing havens for various aquatic plants and aquatic plant communities (e.g., [160]). In Eocene Germany, in the region of Messel, several paleolakes of volcanic origin (maar lakes) are known [161,162]. Regardless of the origin of the lake, being volcanic like the Messel maar or otherwise, they sustained complex ecosystems ranging from deep to shallow waters and from coastline to adjacent terrestrial surroundings (e.g., [163]), providing also ideal aquatic habitats for Stratiotes.
Interestingly, some of the Eocene and Oligocene Stratiotes fossil seeds originate from sediments that accumulated in brackish or shallow marine environments (e.g., [11,160]). The sedimentary context of the fossil African Stratiotes, the Dodori MT from the earliest late Eocene, fits within this scenario. The fossil pollen originated from the Kipini Fm (Figure 2) and was sampled from a sedimentary sequence dominated by sandstones interbedded with various mudstones, claystones, and lignite layers interpreted to have accumulated within a delta-front environment [164]. Further evidence of brackish or estuarine water comes from the co-occurrence of Nypa (“mangrove”) palm pollen in the same sample. We can hypothesize that the African Stratiotes was part of the lowland wetland/aquatic vegetation in East Africa during the Eocene, growing in lagoonal or estuarine bodies close to the coastline associated with paleo-river(s) running eastwards into the ocean.
As mentioned previously, Stratiotes’ current distribution covers Europe and parts of western Central Asia (see [11] Figure 8). In its main distribution area, Western and Central Europe, Stratiotes is thriving under a fully humid warm temperate climate with warm summers (Köppen–Geiger Cfb-climate; cf. [165]) (Supplementary Material S2). At its margins, in parts of Scandinavia, Eastern Europe, and Central Asia, Stratiotes is growing under a fully humid snow climate with warm and/or cool summers and cold winters (Dfb-, Dfc-climates). Taking into account that the European fossil record of Stratiotes extends approximately 56 million years back in time (Supplementary Material S1, Table S2) and that climate in Europe was (much) warmer during most of the Cenozoic than at present (e.g., [166,167]), one can conclude that the current distinctly temperate climate preference of the sole surviving species, S. aloides, is not representative of its generic lineage. Previous studies record between 8 and 16 different Stratiotes species throughout the Cenozoic of Eurasia (e.g., [11,50,81,121,137,160]). During most of the Eocene, when Stratiotes dispersed eastwards across Europe and was represented there by up to five different taxa (e.g., [11,137,160]), the region was in a hot and humid “paratropical” climate comparable to that of the present-day moist tropics to hot subtropics [166,167]. In Europe, this paleoclimatic regime sustained a unique thermophilic flora, the so-called Paratropical Rainforest (e.g., [160]), that disappeared from Europe at the end of the Eocene. The potential modern analogs of many Paratropical Rainforest plant taxa (e.g., [160]) are currently thriving under various equatorial climates (Af-, Am-, Aw-climates) or fully humid warm temperate climates with hot summers (essentially a subtropical Cfa-climate). This means that during its initial European and out-of-Europe radiation, Stratiotes was accustomed to a climate considerably warmer (and more humid) than at present. The climate in Messel, Germany, c. 48 Ma was much warmer than present and located approximately 6° farther south than today, at a paleolatitude of about 45° N (see [161] Figure 2.2). Paleoclimatic estimations based on fossil plant remains from Messel suggest a mean annual temperature ranging from 16.8 to 23.9 °C, with temperatures during summer months reaching 24.7–27.9 °C. The temperature of the coldest month is believed to have exceeded 10 °C, supporting a frost-free climate [168]. Oxygen isotope measurements based on vertebrate fossils from Messel indicate similar paleotemperatures around 18 ± 2.5 °C [169]. The climate in Kenya 38–37 Ma was undoubtedly warmer than present and located approximately 10° farther south than today, at a paleolatitude of about 8° S. A recent reassessment of sea surface temperatures (SSTs) determined from coupled clumped isotope (Δ47) and Mg/Ca measurements on the carbonate from early Eocene foraminifera in cores from off the coast of Tanzania indicate tropical SSTs were between 30 °C and 36 °C throughout the Eocene [170]. Middle Eocene clumped isotope (Δ47)-derived values (at paleolatitude ~19° S) are near the higher end of that range. Although we do not precisely know the land-based equivalent of sea surface temperatures, it was indeed hot. By comparison, the modern mean annual temperature in Lamu, Kenya, is approximately 27 °C. Global climate models produce an enhanced hydrological cycle at low latitudes (e.g., [171]), but this may not be important regarding Stratiotes, an aquatic plant.
3.3. Paleogene Origin and Dispersal of Hydrocharitaceae
Although our dated phylogeny does not retrieve considerably different ages from Chen et al. [9], our biogeographical analysis shows that inferring the ancestral age of Hydrocharitaceae based solely on the distribution of extant taxa is potentially misleading. Indeed, our results support a much more important role for Europe in the distribution of the family. Although this could be partially driven by the excellent European record for the family, which in turn could be driven by collection bias, our analysis did not retrieve major conflicting signals that could have informed the distribution of fossil taxa, whose absence in the other biogeographical areas was left as uncertain. With the exception of the seagrasses, whose distribution is probably not limited by biogeographical barriers, widespread clades such as Najas and Hydrocharis seem to have dispersed out of Europe after the mid-Miocene, while more endemic genera such as Vallisneria, Lagarosiphon, and Elodea sensu lato obtained their current distribution (or close to their current distribution) earlier than the Miocene. This difference in biogeographical history, taken together with the results of our climate signatures, opens the possibility that at least some of the genera of the Hydrocharitaceae were limited in their distribution not by biogeographical barriers, but rather by the availability of appropriate climates. The mid-Miocene transition pushed some of the genera outside of the boreotropics and into the tropical climates of Southeast Asia, Africa, and the Americas, while eradicating them from Europe. On the other hand, the more cold-tolerant forms of Stratiotes persisted at high latitudes, leading to the current distribution of Stratiotes aloides.
4. Material and Methods
4.1. Origin of Samples
4.1.1. Messel, Germany, Europe
The fossil Stratiotes pollen from Messel, Germany (Figure 1), was extracted from a clayey siltstone sample, originally positioned 176 m below the surface (sample nr. 54 in [172]), from core Messel 2001, Messel, Germany. The Messel 2001 core was drilled in 2001 during drilling to a depth of 433 m in the ± central part of the Messel pit to confirm the assumed volcanic origin of the Messel paleolake [20].
4.1.2. Dodori, Kenya, Africa
The fossil Stratiotes pollen from Kenya, Africa (Figure 2), was recovered from a silty mudstone sample, originally positioned 4503 ft (1373 m) below the surface, from core Dodori-1, Lamu Basin, Kenya, Africa. The Dodori-1 core is named after a settlement in Kenya’s Lamu County that is situated 13 miles (21 km) inland from the coast. The Dodori-1 core was produced in 1964 during drilling to a depth of c. 14,140 feet (4340 m) in order to investigate potential hydrocarbon reservoirs in Upper Cretaceous to Cenozoic sedimentary rocks at the coast of the ancient Lamu Embayment, southeast Kenya [164].
4.2. Preparation and Study of Samples
The sedimentary rock samples were processed, and fossil pollen was extracted according to the method explained in Grímsson et al. [173]. Pollen from extant Stratiotes aloides was sampled from herbarium specimens WU0152793 [174], WU0152794 [174], and WU0152795 [174], housed in the University of Vienna Herbarium (WU; Supplementary Material S1, Table S3). The fossil and extant pollen were investigated both by light microscopy (LM) and SEM using the single-grain method as described by Zetter [175] and Halbritter et al. [176] (pp. 121–123), as well as with transmission electron microscopy (TEM) following Ulrich and Grímsson [177]. The SEM stubs and TEM sections with the fossil and extant pollen produced under this study are stored in the collection of the Department of Botany and Biodiversity Research, University of Vienna, Austria. The pollen terminology follows Punt et al. [178] (LM) and Halbritter et al. [176] (LM, SEM, and TEM). For practical reasons, the fossil pollen types are classified as morphotypes (MTs) named after the place/well where they were found.
During pollen preparation for LM, SEM, and TEM, it became clear that fossil and extant Stratiotes pollen grains are incredibly fragile and break and disintegrate easily. Sculpture elements (echini and muri) also fall off easily. This instability is due to the open structure, loosely attached segments comprising the infratectum, and the discontinuity of the footlayer and tectum. The pollen of Stratiotes is also inaperturate, and the pollen wall can rupture anywhere. Already during acetolysis, the pollen grains started to break and it can be assumed that this is part of the reason for the absence of Stratiotes pollen in the geological record.
4.3. Geographic and Geological Background
4.3.1. Messel, Germany, Europe
The Messel pit (Figure 1A,B) is world-famous for the numerous exceptional fossils that have been discovered there since 1875 [179] and include priceless (in a scientific context) plant, invertebrate, and vertebrate remains (e.g., [180,181,182]). The sediments of the Messel pit accumulated during the early and middle Eocene in a crater/maar structure caused by phreatomagmatic activity dated as c. 47.8 Ma [183], c. 48.2 Ma [184], or 48.06 Ma [185]. The crater filling has been divided into four main units based on the Messel 2001 core and previous onsite research [20,182]. At the base, at a depth of 433–228 m, are massive pyroclastics and a diatreme breccia bearing no formal name (Figure 1C). The lake sediments (rocks) overlying the pyroclastics have been divided into the Lower, Middle, and Upper Messel Formation (Fm). The Lower Messel Fm occurs at 228–94 m depth in the core and comprises breccia, sand- to claystone, as well as lapilli tuffs (Figure 1C). The Middle Messel Fm occurs at 94–0 m depth in the core and consists of finely laminated dark olive-grey to dark brownish-grey clay- and siltstones (oil shale) intercalated with few sandy-gravelly layers and occasional layers of siderite (Figure 1C). The Upper Messel Fm, which has been mined away, was allegedly up to 40 m thick and composed of finely laminated blackish claystone intercalated with lignite seams and clayey sands (e.g., [20,182]). The sedimentary sample with the fossil Stratiotes pollen presented herein originates from the Lower Messel Fm, assigned to the Ypresian (early Eocene), and is between 48.27 and 48.05 Ma [184].
4.3.2. Dodori, Kenya, Africa
The Lamu Basin covers a large area of southeastern Kenya and southwestern Somalia with an aerial extent of 132,720 km2 (Figure 2A) [186]. It is the largest basin in Kenya, composed of sedimentary rocks of Permian to Cenozoic age, including continental rift basin sandstones, fluvio-deltaic sandstones, marine shales, and carbonates. The Cretaceous and Cenozoic sediments have been divided into three megasequences deposited under alternating periods of transgression and regression, thus incorporating unconformities of regional and local significance [164]. Megasequence II comprises the rocks deposited between the late Jurassic and the late Paleocene, also defined as the Sabaki Group. Megasequence III constitutes the Tana Group, comprising, among others, the Kipini Fm and the Dodori limestone (Figure 2B), which are Paleogene deposits accumulated during three pulses of sea-level rise and a single regressive phase. Megasequence IV (Coastal Group) is defined by an unconformity that separates the underlying late Oligocene strata of the Tana Group from the youngest sediments filling the basin [164]. The Kipini Fm comprises several sandstones interbedded with claystones and mudstones, and the Dodori limestone is an expression of the shelf carbonate deposition that occurred in the area until the late Eocene [164]. The studied sample originates from a greyish shale of the Kipini Fm, positioned just below the Dodori limestone (at 4503 ft (1373 m) below the surface; Figure 2B), and is of earliest late Eocene (earliest Priabonian) age (~37.71 Ma; following [187]). The consistent occurrence of the large foraminifera, Nummulites fabianii Prever (from 4410 ft (1344 m) upwards), as well as various dinoflagellate cysts (including Cordosphaeridium fibrospinosum R.J.Davey & G.L.Williams) characteristic of the late Eocene, support the Priabonian age of the sedimentary matrix containing the fossil Stratiotes pollen. Generally, middle-to-late Eocene sediments in this region consist of interbedded nummulitic sands, poorly sorted and fine to very coarse-grained calcareous sandstones, nummulitic and micritic limestones, and dark olive greenish grey shales and grey-green silty mudstones. The sample containing the fossil Stratiotes pollen originates from the top of a sedimentary rock unit interpreted to have been deposited within a delta-front environment (Figure 2B) [164].
4.4. Dated Phylogeny
We inferred a dated phylogeny of the Hydrocharitaceae using the molecular matrix of Bernardini and Lucchese [10]. This included one nuclear locus (ITS) and five plastid loci (matK, rbcl, rpoB, rpoC, and trnK) for 50 species of Hydrocharitaceae, including the genera Blyxa (3/14 species sampled), Elodea (5/9, including 1 species formerly in Agapanthe Planch. and 2 species formerly in Egeria), Enhalus (1/1), Halophila (4/17), Hydrilla (1/1), Hydrocharis (4/5, including 2 species formerly in Limnobium), Lagarosiphon (3/9), Najas (10/39), Nechamandra (1/1), Ottelia (5/23), Stratiotes (1/1), Thalassia (1/2), and Vallisneria (10/16, including 1 species formerly in Maidenia Rendle). This dataset covers all the genera of the Hydrocharitaceae, with the exclusion of the monotypic Appertiella C.D.K.Cook & L.Triest, for which no molecular data are available.
After trimming one unidentified species and using Butomus umbellatus L. as the only outgroup, we used the resulting alignment in a tip-dating analysis under the Fossilized Birth-Death prior as employed in the software MrBayes v. 3.2.7 [188] as implemented in the CIPRES science gateway [189]. Fossil taxa of Hydrocharitaceae were added and constrained to their more likely position (see Supplementary Material S1, Table S4). The prior on the age of the root was set between 113 Ma and 56 Ma, corresponding to the oldest unequivocal fossil evidence of the monocots [82] and the oldest age of the older fossil, respectively. We employed two unlinked clock models, one for the plastid markers and one for the ITS, using an uncorrelated log-normal clock. Tip ages were implemented as uniform distributions to improve accuracy [190]. The proportion of extant sampling was set to 0.37. The MCMC was run for 50,000,000 generations, sampling every 5000 generations. Convergence was assessed using the software Tracer [191], checking for ESS of more than 200. A Maximum Clade Credibility (MCC) tree was generated discarding the first 10% of the posterior trees as burn-in. The tree was then plotted using the R package MCMCtreeR.
4.5. Biogeographical Analysis
A biogeographical analysis was conducted using the DEC model implemented in the R package BioGeoBEARS [192] using the MCC tree from the Bayesian analysis. We conducted the analyses using the biogeographical areas used by Chen et al. [9]. Area occupancy of the different species was scored based on the distribution retrieved from the Plants of the World Online database [26]. For the area occupancy of the fossils, we used the option “useAmbiguities = TRUE” in BioGeoBEARS, to allow the areas where the fossil was not sampled from to be scored as unknown, to reflect the uncertainty over the true absence of fossil taxa across their potential distribution.
4.6. Climate Data Harvesting and Analysis
We used Köppen profiles (e.g., [193,194,195,196,197]) to summarize the climatic niches occupied by extant Hydrocharitaceae species/genera and to hypothesize about their climatic niche evolution (Supplementary Material S2). A Köppen profile reflects the proportional Köppen–Geiger climate (cf. [165,198]) zone coverage of modern plant species based on their gridded distribution data from GBIF.org (Supplementary Material S1, Table S5). Unfortunately, no distribution data were available on GBIF.org for the genus Appertiella and some species of Blyxa, Ottelia, Hydrocharis, Hydromystria G.Mey., Halophila, Thalassia, Najas, and Vallisneria. Modern species distributions were checked for outliers (e.g., neophyte distribution) using published chorological data (e.g., [26]). Additionally, multiple occurrences with identical coordinates were merged (labeled ‘unique localities’ in the diagrams (Supplementary Material S2). The revised georeferenced occurrence data were then plotted onto 1 km2 grid Köppen–Geiger maps (1979–2013 data; [27]) to establish Köppen profiles for all modern species/genera. The georeferenced data and the Köppen–Geiger maps with 1 km2 resolution were processed using the ‘Sample Raster Values’ Toolbox in Qgis v.3.16.4-Hannover. The Köppen–Geiger climates occupied by extant Hydrocharitaceae species/genera are shown as maps generated in Qgis and as frequency (proportional distribution) diagrams (Supplementary Material S2). To simplify interpretations, the Köppen profiles of Hydrocharitaceae genera are summarized into five climatic niches. Additionally, precipitation (second letter in Köppen climate types) was excluded since all members of Hydrocharitaceae are aquatic and, therefore, not susceptible to rainfall seasonality/humidity availability.
5. Conclusions and Future Considerations
The oldest Stratiotes fossils are seeds from the early Eocene of England (London Clay), and the oldest unequivocal pollen is from the early Eocene of Germany (Messel) and the late Eocene of equatorial Kenya (Dodori, Africa). The Eocene is known as the global warm house [199], meaning the oldest known Stratiotes fossils were preserved under tropical or paratropical conditions. Therefore, it can be assumed that the ancestral climatic signal for Stratiotes, and Hydrocharitaceae in general, was tropical to hot/warm temperate. We assume that the African lineages of Stratiotes went extinct while the European lineages diversified and adapted to the changing climate during the Oligocene and Neogene, surviving the transition from a hot/warmhouse to a coolhouse climate. The ice ages probably presented a final bottleneck in reducing the lineage’s diversity to the remaining monotypic Stratiotes aloides.
The fossil Stratiotes pollen described herein is unique, and the only reliable pre-Holocene pollen record of this genus so far. The Stratiotes pollen from the Messel pit is the first unequivocal fossil record representing this genus from this locality. This finding underlines the potentiality of re-investigating European paleopalynofloras using combined LM and SEM, which have until now mostly been investigated using conventional LM.
Despite the size of the African continent, it has revealed few Cretaceous to Cenozoic macro- and mesofloras [28]. This places African palynofloras on a pedestal and makes them the most important paleobotanical source for resolving paleophytogeographic distribution patterns and interpreting past vegetation and climate changes in this part of the globe. The unique fossil Stratiotes pollen from Kenya (Africa) presented herein again demonstrates the usefulness of the single-grain method for paleopalynology (e.g., [200,201,202]). Future work on Africa’s palynofloras must include combined LM and SEM studies. The African paleopalynoflora is a closed treasure chest, and the single-grain method is the key to opening it. Routinely applying this method when analyzing Cretaceous and Cenozoic palynofloras from this part of the globe will certainly cast new light on the origin and evolution of Africa’s forests and vegetation units.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants13071008/s1. Supplementary Material S1. This Excel file comprises Tables S1–S6 (and references therein, Table S7) referred to in the main text [11,12,22,24,25,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,160,180,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217]. Supplementary Material S2. This PDF comprises distribution and climate data for Hydrocharitaceae in a phylogenetic context [9,27,165,198,218]. Supplementary Material S3. This PDF comprises additional LM, SEM, and TEM micrographs of extant Stratiotes aloides pollen. Supplementary Material S4. This PDF comprises Bayesian and IQTREE produced for this study.
Author Contributions
Conceptualization, S.U. and F.G.; Methodology, S.U., F.G. and M.C.; Investigation, S.U., M.V., M.C., J.M.B., C.G., B.F.J., E.D.C., O.K.L., V.W., R.Z. and F.G.; Data Curation, S.U., F.G., J.M.B. and M.C.; Writing—Original Draft Preparation, F.G., M.V. and M.C.; Writing—Review and Editing, S.U., M.V., M.C., J.M.B., C.G., B.F.J., E.D.C., O.K.L., V.W., R.Z. and F.G.; Supervision, F.G.; Project Administration, F.G.; Funding Acquisition, F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Austrian Science Fund (FWF) with a grant to F.G., project number P34303; Open Access Funding by the University of Vienna. For the purpose of open access, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission.
Data Availability Statement
All data supporting the results are either provided within the manuscript or in the Supplementary Materials.
Acknowledgments
We thank Guido Grimm, Orléans, France, for the critical evaluation of published molecular phylogenetic trees and biogeographic analyses regarding Hydrocharitaceae. We thank Open Access Funding by the University of Vienna.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Taylor, T.N.; Taylor, E.L.; Krings, M. Paleobotany—The Biology and Evolution of Fossil Plants; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. Early Flowers and Angiosperm Evolution; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linnéan Soc. 2003, 141, 399–436. [Google Scholar] [CrossRef]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linnéan Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linnéan Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Stevens, P. Angiosperm Phylogeny Website, Version 14. 2017. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 15 December 2023).
- Tanaka, N.; Setoguchi, H.; Murata, J. Phylogeny of the Family Hydrocharitaceae Inferred from rbcL and matK Gene Sequence Data. J. Plant Res. 1997, 110, 329–337. [Google Scholar] [CrossRef]
- Les, D.H.; Moody, M.L.; Soros, C.L. A reappraisal of phylogentic relationships in the monocotyledon family Hydrocharitaceae (Alismatidae). Aliso J. Syst. Florist. Bot. 2006, 22, 211–230. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Chen, J.-M.; Gituru, R.W.; Wang, Q.-F. Generic phylogeny, historical biogeography and character evolution of the cosmopolitan aquatic plant family Hydrocharitaceae. BMC Evol. Biol. Biol. 2012, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, B.; Lucchese, F. New phylogenetic insights into Hydrocharitaceae. Ann. Bot. 2018, 8, 45–58. [Google Scholar] [CrossRef]
- Cook, C.D.K.; Urmi-König, K. A revision of the genus Stratiotes (Hydrocharitaceae). Aquat. Bot. 1983, 16, 213–249. [Google Scholar] [CrossRef]
- Cook, C. Hydrocharitaceae. In The Families and Genera of Vascular Plants. Volume 4. Flowering Plants. Monocotyledons. Alismatanae and Commelinanae (Except Gramineae); Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 234–248. [Google Scholar]
- Katzenberger, J.; Zacharias, D. Mutualism of Stratiotes aloides L. (Hydrocharitaceae) and Hydrellia tarsata Haliday (Diptera: Ephydridae): Tritrophic interaction of macrophyte, leaf-mining dipteran pollinator and parasitoid Braconidae. J. Pollinat. Ecol. 2015, 15, 23–29. [Google Scholar] [CrossRef]
- Daumann, E. Zur Morphologie und Ökologie der Blüte von Stratiotes aloides L. Planta 1931, 14, 766–776. [Google Scholar] [CrossRef]
- Efremov, A.N.; Filonenko, A.V.; Sviridenko, B.F. Anatomy and morphology of reproductive organs of Stratiotes aloides L. (Hydrocharitaceae). Inland Water Biol. 2015, 8, 334–344. [Google Scholar] [CrossRef]
- Sun, K.; Chen, J.-K.; Zhang, Z.-Y. Pollen morphology of 15 species in nine genera of the Hydrocharitaceae. Acta Phytotaxon. Sin. 2002, 40, 490–500. [Google Scholar]
- Tanaka, N.; Uehara, K.; Murata, J. Correlation between pollen morphology and pollination mechanisms in the Hydrocharitaceae. J. Plant Res. 2004, 117, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Toma, C. Reproduction of Stratiotes aloides in Ilmoilanselkä Lake (Finland). J. Ecol. Prot. Coastline 2012, 16, 103–111. [Google Scholar]
- Harms, F.-J.; Aderhold, G.; Hoffmann, I.; Nix, T.; Rosenberg, F. Erläuterungen zur Grube Messel bei Darmstadt (Südhessen). Schriftenreihe Dtsch. Geol. Ges. 1999, 8, 181–222. [Google Scholar]
- Felder, M.; Harms, F.J.; Wolf, K. Lithologie und genetische Interpretation der vulkano-sedimentären Ablagerungen aus der Grube Messel anhand der Forschungsbohrung Messel 2001 und weiterer Bohrungen. CFS Cour. Forschungsinstitut Senckenb. 2004, 252, 151–203. [Google Scholar]
- Vieira, M.; Bouchal, J.M.; Geier, C.; Ulrich, S.; Zetter, R.; Grímsson, F. Earliest fossil pollen records of endemic African Sclerosperma palms and the palaeoecological aspects of the genus. Rev. Palaeobot. Palynol. 2023, 317, 104954. [Google Scholar] [CrossRef]
- Wilde, V. Untersuchungen zur Systematik der Blattreste aus dem Mitteleozän der Grube Messel bei Darmstadt-(Hessen, Bundesrepublik Deutschland). CFS Cour. Forschungsinstitut Senckenb. 1989, 115, 1–213. [Google Scholar]
- Collinson, M.E.; Manchester, S.R.; Wilde, V. Fossil Fruits and Seeds of the Middle Eocene Messel biota, Germany. Abh. Senckenb. Ges. Naturforsch. 2012, 570, 1–251. [Google Scholar]
- Thiele-Pfeiffer, H. Die Mikroflora aus dem mitteleozänen Ölschiefer von Messel bei Darmstadt. Palaeontogr. Abt. B 1988, 211, 1–86. [Google Scholar]
- Bouchal, J.M.; Geier, C.; Ulrich, S.; Wilde, V.; Lenz, O.K.; Zetter, R.; Grímsson, F. Qualitative LM and SEM study of the Messel palynoflora: Part I. Algae to Vitales. Grana submitted. 2024.
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 1 February 2024).
- Cui, D.; Liang, S.; Wang, D.; Liu, Z. A 1 km global dataset of historical (1979–2013) and future (2020–2100) Köppen–Geiger climate classification and bioclimatic variables. Earth Syst. Sci. Data 2021, 13, 5087–5114. [Google Scholar] [CrossRef]
- Jacobs, B.F.; Pan, A.D.; Scotese, C.R.; Werdelin, L.; Sanders, W.J. A.D.; Scotese, C.R.; Werdelin, L.; Sanders, W.J. A review of the Cenozoic vegetation history of Africa. In Cenozoic Mammals of Africa; University of California Press: Berkeley, CA, USA, 2010; pp. 57–72. [Google Scholar]
- Turner, B.; Hameister, S.; Hudler, A.; Bernhardt, K. Genetic Diversity of Stratiotes aloides L. (Hydrocharitaceae) Stands across Europe. Plants 2021, 10, 863. [Google Scholar] [CrossRef]
- Zetter, R.; Hofmann, C.-C. New aspects of the palynoflora of the lowermost Eocene (Krappfeld area, Carinthia). Osterr. Akad. Wiss. Schiftenr. Erdwissenschaftlichen Komm. 2001, 14, 473–507. [Google Scholar]
- Ridley, H.N. The Dispersal of Plants throughout the World; L. Reeve & Co. Ltd.: Ashford, UK, 1930. [Google Scholar]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.-F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef]
- Grímsson, F.; Graham, S.A.; Coiro, M.; Jacobs, B.F.; Xafis, A.; Neumann, F.H.; Scott, L.; Sakala, J.; Currano, E.D.; Zetter, R. Origin and divergence of Afro-Indian Picrodendraceae: Linking pollen morphology, dispersal modes, fossil records, molecular dating and paleogeography. Grana 2019, 58, 227–275. [Google Scholar] [CrossRef]
- Grímsson, F.; Kapli, P.; Hofmann, C.C.; Zetter, R.; Grimm, G.W. Eocene Loranthaceae pollen pushes back divergence ages for major splits in the family. PeerJ 2017, 5, e3373. [Google Scholar] [CrossRef]
- Grímsson, F.; Xafis, A.; Neumann, F.H.; Scott, L.; Bamford, M.K.; Zetter, R. The first Loranthaceae fossils from Africa. Grana 2018, 57, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, T.L.P.; Dauby, G.; Blach-Overgaard, A.; Deblauwe, V.; Dessein, S.; Droissart, V.; Hardy, O.J.; Harris, D.J.; Janssens, S.B.; Ley, A.C.; et al. Tectonics, climate and the diversification of the tropical African terrestrial flora and fauna. Biol. Rev. 2021, 96, 16–51. [Google Scholar] [CrossRef]
- Rage, J.-C.; Gheerbrant, E. Island Africa and vertebrate evolution: A review of data and working hypotheses. In Biological Consequences of Plate Tectonics: New Perspectives on Post-Gondwana Break-Up—A Tribute to Ashok Sahni; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 251–264. [Google Scholar]
- Baas, J. Eine frühdiluviale Flora im Mainzer Becken. Z. Bot. 1932, 25, 289–371. [Google Scholar]
- Bennike, O.; Hoek, W. Late Glacial and early Holocene records of Stratiotes aloides L. from northwestern Europe. Rev. Palaeobot. Palynol. 1999, 107, 259–263. [Google Scholar] [CrossRef]
- Benzecry, A.; Brack-Hanes, S.D. A new hydrocharitacean seagrass from the Eocene of Florida. Bot. J. Linn. Soc. 2008, 157, 19–30. [Google Scholar] [CrossRef]
- Bogner, J.; Kvaček, Z. A fossil Vallisneria plant (Hydrocharitaceae) from the early Miocene freshwater deposits of the Most Basin (North Bohemia). Aquat. Bot. 2009, 90, 119–123. [Google Scholar] [CrossRef]
- Bohncke, S.J.P. Lateglacial environmental changes in the Netherlands: Spatial and temporal patterns: A contribution to the ‘North Atlantic seaboard programme’ of IGCP-253, ‘Termination of the Pleistocene’. Quat. Sci. Rev. 1993, 12, 707–717. [Google Scholar] [CrossRef]
- Bohncke, S.; Wijmstra, L.; Van Der Woude, J.; Sohl, H. The Late-Glacial infill of three lake successions in The Netherlands: Regional vegetational history in relation to NW European vegetational developments. Boreas 1988, 17, 385–402. [Google Scholar] [CrossRef]
- Bohncke, S.; Vandenberghe, J.; Huijzer, A.S. Periglacial environments during the Weichselian Late Glacial in the Maas valley, the Netherlands. Geol. Mijnb. 1993, 72, 193–210. [Google Scholar]
- Bohncke, S.; Kasse, C.; Vandenberghe, J. Climate induced environmental changes during the Vistulian Lateglacial at Zabinko, Poland. Quaest. Geogr. 1995, 4, 43–64. [Google Scholar]
- Bone, D.A. The stratigraphy of the Reading beds (Palaeocene), at Felpham, West Sussex. Tert. Res. 1986, 8, 17–32. [Google Scholar]
- Brack-Hanes, S.D.; Greco, A.M. Biomineralization in Thalassia testudinum (Liliopsida: Hydrocharitaceae) and an Eocene seagrass. Trans. Am. Microsc. Soc. 1988, 107, 286–292. [Google Scholar] [CrossRef]
- Bůžek, Č. Přispěvek k poznáni flóry panonu v Poštorné u Břeclavi. Čas. Miner. Geol. 1962, 7, 257–259. [Google Scholar]
- Cantemir, V.V.; Negru, A.G. Novye pozdnemiotsenovye vidy rastenij roda Najas L. Bul. Acad. Ştiinţe R.S.S. Mold. 1989, 2, 8–11. [Google Scholar]
- Chandler, M.E.J. The geological history of the genus Stratiotes: An account of the evolutionary changes which have occurred within the genus during Tertiary and Quaternary times. Q. J. Geol. Soc. 1923, 79, 117–138. [Google Scholar] [CrossRef]
- Chandler, M.E.J. The Upper Eocene Flora of Hordle, Hants. Part I. Monogr. Palaeontogr. Soc. 1925, 77, 1–32. [Google Scholar] [CrossRef]
- Chandler, M.E.J. Plant remains of the Hengistbury and Barton Beds. Bull. Br. Mus. Nat. Hist. Geol. 1960, 4, 193–238. [Google Scholar] [CrossRef]
- Chandler, M.E.J. The Lower Tertiary Floras of Southern England. III. Flora of the Bournemouth Beds; the Boscombe, and the Highcliff Sands; British Museum (Natural History): London, UK, 1963. [Google Scholar]
- Chandler, M.E.J. The Lower Tertiary Floras of Southern England. IV. A Summary and Survey of Findings in the Light of Recent Botanical Observations; British Museum (Natural History): London, UK, 1964. [Google Scholar]
- Cleveringa, P.; De-Gans, W.; Kolstrup, E.; Paris, F.P. Vegetational and climatic developments during the Late Glacial and the early Holocene and aeolian sedimentation as recorded in the Uteringsvee (Drente, The Netherlands). Geol. Mijnb. 1977, 56, 234–242. [Google Scholar]
- Collinson, M.E.; Cleal, C.J. The palaeobotany of the Palaeocene and Palaeocene-Eocene transitional strata in Great Britain. Geol. Conserv. Rev. Ser. 2001, 22, 155–184. [Google Scholar]
- Collinson, M.E. Fruit and seed floras from the Palaeocene/Eocene transition and subsequent Eocene in southern England: Comparison and palaeoenvironmental implications. GFF 2000, 122, 36–37. [Google Scholar] [CrossRef]
- Collinson, M.E.; Fowler, K.; Boulter, M.C. Floristic changes indicate a cooling climate in the Eocene of southern England. Nature 1981, 291, 315–317. [Google Scholar] [CrossRef]
- Collinson, M.E.; Hooker, J.J.; Gröcke, D.R. Cobham lignite bed and penecontemporaneous macrofloras of southern England: A record of vegetation and fire across the Paleocene-Eocene Thermal Maximum. Geol. Soc. Am. Spec. Pap. 2003, 369, 333–350. [Google Scholar]
- Čtyroký, P.; Knobloch, E. Neue paläontologische Untersuchungen im Pannon des NO-Teils des Wiener Beckens. Časopis Morav. Muz. Brné Vĕdy Přírodni 1976, 61, 97–114. [Google Scholar]
- Daley, B.; Edwards, N. The Bembridge Limestone (Late Eocene), Isle of Wight, southern England: A stratigraphical revision. Tert. Res. 1990, 12, 51–64. [Google Scholar]
- Daley, B. The palaeoenvironment of the Bembridge Marls (Oligocene) of the Isle of Wight, Hampshire. Proc. Geol. Assoc. 1973, 84, 83–93. [Google Scholar] [CrossRef]
- Denk, T.; Güner, T.H.; Kvaček, Z.; Bouchal, J.M. The early Miocene flora of Güvem (Central Anatolia, Turkey): A window into early Neogene vegetation and environments in the Eastern Mediterranean. Acta Palaeobot. 2017, 57, 237–338. [Google Scholar] [CrossRef]
- Dixon, F.S. Paleoecology of an Eocene Mud-Flat Deposit (Avon Park Formation, Claibornian) in Florida. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1972. [Google Scholar]
- Dorofeev, P.I. On the systematics of the fossil Najads of the subgenus Caulinia (Willd.) Aschers. Bot. Zhurnal 1973, 58, 385–394. [Google Scholar]
- Dorofeev, P.I. Tretichnye Flory Zapadnoj Sibiri; Izdateľstvo Akademii Nauk SSSR: Moscow, Russia, 1963. [Google Scholar]
- Dorofeev, P.I. Novye Dannye o Pliotsenovoj Flore Bashkirii. In Stratigrafija Chetvertichnykh (Antropogenovykh) Otlozhenij Urala; Yakhimovich, V.L., Lider, V.A., Eds.; Izdatel’stvo “Nedra”: Moscow, Russia, 1965; pp. 191–219. [Google Scholar]
- Dorofeev, P.I. Pliotsenovaja Flora Matanova Sada Na Donu; Izdatel’stvo “Nauka”, Leningradskoe otdelenie: Leningrad, Russia, 1966. [Google Scholar]
- Edwards, N.; Daley, B. Stratigraphy of the Totland Bay Member (Headon Hill Formation, Late Eocene) at Hordle Cliff, Hampshire, southern England. Tert. Res. 1997, 18, 35–50. [Google Scholar]
- Friis, E.M. Angiosperm fruits and seeds from the Middle Miocene of Jutland (Denmark). K. Dan. Vidensk. Selsk. Biol. Skr. 1985, 24, 1–165. [Google Scholar]
- Gotjé, W. De Holocene Laagveenontwikkeling in de Randzone van de Nederlandse Kustvlakte (Noordoostpolder); Vrije Universiteit: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Grambast, L. Flore de l’Oligocène supérieur du Bassin de Paris. Ann. Paléontologie 1962, 48, 85–126. [Google Scholar]
- Gregor, H.-J. Trapa zapfei Berger aus dem Untermiozän von Langau bei Geras (NÖ.)—Eine Hydrocharitacee. Ann. Naturhistorischen Mus. Wien 1980, 88, 105–118. [Google Scholar]
- Gregor, H.J. Contributions to the late Neogene and early Quaternary floral history of the Mediterranean. Rev. Palaeobot. Palynol. 1990, 62, 309–338. [Google Scholar] [CrossRef]
- Hantke, R. Die fossile Flora der obermiozänen Oehninger-Fundstelle Schrotzburg (Schienerberg, Süd-Baden). Denkschr. Schweiz. Naturforschenden Ges. 1954, 80, 27–118. [Google Scholar]
- Hartz, N. Bidrag til Danmarks tertiære og diluviale Flora. Dan. Geol. Undersøgelse II Række 1909, 20, 1–292. [Google Scholar]
- Hayes, P.A.; Collinson, M.E. The flora of the Insect Limestone (latest Eocene) from the Isle of Wight, southern England. Earth Environ. Sci. Trans. R. Soc. Edinb. 2014, 104, 245–261. [Google Scholar] [CrossRef]
- Heer, O. Die Tertiäre Flora Der Schweiz.—Flora Tertiara Helvetiae Vol 1; Verlag Wurster & Co.: Winterthur, Switzerland, 1855. [Google Scholar]
- Heer, O. Die Tertiäre Flora Der Schweiz.—Flora Tertiara Helvetiae Vol 2; Verlag Wurster & Co.: Winterthur, Switzerland, 1856. [Google Scholar]
- Henniger, M.; Leder, R.M.; Müller, A. Paläogene Fossilien aus einer Karstschlotte im Unteren Muschelkalk von Karsdorf an der Unstrut (Sachsen-Anhalt, Burgenlandkreis). Z. Dtsch. Ges. Geowiss. 2011, 162, 317–332. [Google Scholar]
- Holý, F.; Bůžek, C. Seeds Stratiotes L. (Hydrocharitaceae) in the Tertiary of Czechoslovakia. Sborník Geol. Věd Paleontol. 1966, 8, 105–135. [Google Scholar]
- Iles, W.J.D.; Smith, S.Y.; Gandolfo, M.A.; Graham, S.W. Monocot fossils suitable for molecular dating analyses. Bot. J. Linn. Soc. 2015, 178, 346–374. [Google Scholar] [CrossRef]
- Ivany, L.C.; Portell, R.W.; Jones, D.S. Animal-plant relationships and paleobiogeography of an Eocene seagrass community from Florida. Palaios 1990, 5, 244–258. [Google Scholar] [CrossRef]
- Jakubovskaja, T.V. Ranneantrapagenavyya Nasennyya Flory Belaruskaj Grady i Ikh Stratygrafichnae Stanovishcha. In Dasledavanni antrapagenu Belarusi; Kuzniatsow, U.A., Ed.; Navuka i Tekhnika: Minsk, Russia, 1978; pp. 93–105. [Google Scholar]
- Junge, F.W.; Dolezych, M.; Walther, H.; Böttger, T.; Kühl, A.; Kunzmann, L.; Morgenstein, P.; Steinberg, T.; Stange, R. Ein Fenster in Landschaft und Vegetation vor 37 Millionen Jahren: Lithologische, sedimentgeochemische und paläobotanische Befunde aus einem Paläoflusssystem des Weißelsterbeckens. Mauritiana 2005, 19, 185–273. [Google Scholar]
- Juzepczuk, S.V.S. Najadovye-Najadaceae Benth. et Hook. F. In Flora SSSR I; Komarov, V.L., Ed.; Izdateľstvo Akademii Nauk SSSR: Leningrad, Russia, 1934; pp. 269–275. [Google Scholar]
- Kirchheimer, F. Über die botanische Zugehörigkeit weiterer Früchte und Samen aus dem deutschen Tertiär. Planta 1936, 25, 481–490. [Google Scholar] [CrossRef]
- Kirchheimer, F. Die Laubgewächse der Braunkohlenzeit; Veb Wilhelm Knapp Verlag: Halle, Germany, 1957. [Google Scholar]
- Knobloch, E. Tertiäre Floren von Mähren; Moravské Museum: Brno, Czech Republic, 1969. [Google Scholar]
- Knobloch, E. Samen und Früchte aus dem Pannon von Kunovice (Mähren). Věstník Ústředního Ust. Geol. 1976, 51, 221–230. [Google Scholar]
- Knobloch, E. Die untermiozäne Flora von Šafov in Südmähren. Věstník Ústředního Ust. Geol. 1978, 53, 153–162. [Google Scholar]
- Knobloch, E. Neue paläontologische Untersuchungen im Pannon und Pont des mährischen Teils des Wiener Beckens. Sborník Národního Muz. Praze Řada B 1981, 37, 205–227. [Google Scholar]
- Knobloch, E. Svrchnomiocénní flóra z Bernartic u Javorníka na severní Moravĕ. Časopis Slez. Zemského Muz. Série A 1982, 31, 249–264. [Google Scholar]
- Knobloch, E. Biometrie und Morphologie der Samen von Stratiotes kaltennordheimensis und S. tuberculatus aus dem mitteleuropäischen Neogen. Sborník Geol. Věd Paleontol. 1989, 30, 159–173. [Google Scholar]
- Kräusel, R. Pflanzenreste aus den diluvialen Ablagerungen im Ruhr-Emscher-Lippe-Gebiete. Decheniana 1937, 95A, 207–240. [Google Scholar]
- Kvaček, Z.; Hurník, S. Revision of Early Miocene plants preserved in baked rocks in the North Bohemian Tertiary. Sborník Národního Muz. Praze. Řada B Přírodní Vědy 2000, 56, 1–48. [Google Scholar]
- Kvaček, Z. The Hydrocharitaceae foliage from the North Bohemian early miocene. Věstník Českého Geol. Ust. 1995, 70, 21–27. [Google Scholar]
- Kvaček, Z. Bílina: A window on Early Miocene marshland environments. Rev. Palaeobot. Palynol. 1998, 101, 111–123. [Google Scholar] [CrossRef]
- Kvaček, Z. Aquatic angiosperms of the early Miocene Most Formation of North Bohemia (Central Europe). Cour. Forschungsinstitut Senckenb. 2003, 241, 255–279. [Google Scholar]
- Kvaček, Z.; Böhme, M.; Dvořák, Z.; Konzalová, M.; Mach, K.; Prokop, J.; Rajchl, M. Early Miocene freshwater and swamp ecosystems of the Most Basin (northern Bohemia) with particular reference to the Bílina Mine section. J. Czech Geol. Soc. 2004, 49, 1–40. [Google Scholar]
- Laurent, L. Flora Des Calcaires de Célas; Moullot Fils: Marseille, France, 1899. [Google Scholar]
- Ludwig, R. Fossile Pflanzen aus der ältesten Abtheilung der Rheinisc–Wetterauer Tertiär-Formation. Palaeontographica 1859, 8, 39–72. [Google Scholar]
- Lumber, S.H.; den Hartog, C.; Phillips, R.C.; Olsen, F.S. The occurrence of fossil seagrasses in the Avon Park Formation (late middle Eocene), Levy County, Florida (USA). Aquat. Bot. 1984, 20, 121–129. [Google Scholar] [CrossRef]
- Mai, D.H.; Walther, H. Die Floren der Haselbacher Serie im Weßelster-Becken (Bezirk Leipzig, DDR). Abh. Staatl. Mus. Mineral. Geol. Dresd. 1978, 28, 1–200. [Google Scholar]
- Mai, D.H.; Walther, H. Die pliozänen Floren von Thürigen, Deutsche Demokratische Republic. Quartärpaläontologie 1988, 7, 55–297. [Google Scholar]
- Mai, D.H.; Walther, H. Die oligozänen und untermiozänen Floren Nordwest-Sachsens und des Bitterfelder Raumes. Abh. Staatl. Mus. Mineral. Geol. Dresd. 1991, 38, 1–230. [Google Scholar]
- Mai, D.H.; Walther, H. Die obereozänen Floren des Weißelster-Beckens und seiner Randgebiete. Abh. Staatl. Mus. Mineral. Geol. Dresd. 1985, 33, 1–260. [Google Scholar]
- Mai, D.H. Neue Arten nach Früchten und Samen aus dem Tertiär von Nordwestsachsen und der Lausitz. Feddes Repert. 1987, 98, 105–126. [Google Scholar] [CrossRef]
- Mai, D.H. Tertiäre Vegetationsgeschichte Europas; Gustav Fischer Verlag: Stuttgart, Germany, 1995. [Google Scholar]
- Mai, D.H. Die oberoligozänen Floren am Nordrand der Sächsischen Lausitz. Palaeontogr. Abt. B 1997, 244, 1–124. [Google Scholar]
- Mai, D.H. Die untermiozänen Floren aus der Spremberger Folge und dem 2. Flözhorizont in der Lausitz Teil I: Farnpflanzen, Koniferen und Monokotyledonen. Palaeontogr. Abt. B 1999, 250, 1–76. [Google Scholar] [CrossRef]
- Mai, D.H. Die mittelmiozänen und obermiozänen Floren aus der Meuroer und Raunoer Folge in der Lausitz Teil I: Farnpflanzen, Koniferen und Monokotyledonen. Palaeontogr. Abt. B 2000, 256, 1–68. [Google Scholar] [CrossRef]
- Massalongo, A.B. Plantae Fossiles Novae in Formationibus Tertiariis Regni Veneti Nuper Inventae; Ramanzini: Verona, Italy, 1853. [Google Scholar]
- Meller, B. Systematisch-taxonomische Untersuchungen von Karpo-Taphocoenosen des Köflach-Voitsberger Braunkohlenrevieres (Steiermark, Österreich; Untermiozän) und ihre paläoökologische Bedeutung. Jahrb. Geol. Bundesanst. 1998, 140, 497–655. [Google Scholar]
- Miller, J.A. Hydrogeologic Framework of the Floridan Aquifer System in Florida and Parts of Georgia, Alabama, and South Carolina; Department of the Interior, US Geological Survey: Washington, DC, USA, 1986; Volume 1403. [Google Scholar]
- Nikitin, P.A. Akvitanskaja Semennaja Flora Lagernogo Sada (Tomsk); Izdatel’stvo Tomskogo Universiteta: Tomsk, Russia, 1965. [Google Scholar]
- Nikitin, V.P. Paleokarpologija i Stratigrafija Paleogena i Neogena Aziatskoj Rossii; Akademicheskoe izdatel’stvo “Geo”: Novosibirsk, Russia, 2007. [Google Scholar]
- Pais, J. Contribuição Para o Conhecimento da Vegetação Miocénica da Parte Ocidental da Bacia do Tejo; Universidade Nova de Lisboa: Lisbon, Portugal, 1981. [Google Scholar]
- Palamarev, E. Fossile Floren aus drei Braunkohlebecken in Südwestbulgarien. Izv. Bot. Inst. 1970, 20, 35–79. [Google Scholar]
- Palamarev, E. Fosilnata flora na vuglenosniya eotsen v Burgasko. Izv. Bot. Inst. 1973, 24, 75–124. [Google Scholar]
- Palamarev, E. Die Gattung Stratiotes L. in der Tertiärflora Bulgariens und ihre Entwicklungsgeschichte in Eurasien. Phytology 1979, 12, 3–36. [Google Scholar]
- Palamarev, E. Paläokarpologische Untersuchungen des Braunkohlenjungtertiärs in Bulgarien. Palaeontogr. Abt. B Paläophytologie 1994, 232, 129–154. [Google Scholar]
- Palamarev, E.; Ivanov, D.; Kitanov, G. New data about the fossil flora from Bobovdol basin and its biostratigraphic significance. Spis. Bălgarskoto Geol. 1998, 59, 13–21. [Google Scholar]
- Palamarev, E.H.; Bozukov, V.; Uzunova, K.; Petkova, A.; Kitanov, G. Catalogue of the Cenozoic plants of Bulgaria (Eocene to Pliocene). Phytol. Balc. 2005, 11, 215–364. [Google Scholar]
- Paris, F.P.; Cleveringa, P.; de Gans, W. The Stokersdobbe: Geology and palynology of a deep pingo remnant in Friesland (The Netherlands). Geol. Mijnb. 1979, 58, 33–38. [Google Scholar]
- Rasmussen, E.S.; Dybkjær, K.; Piasecki, S. Lithostratigraphy of the upper Oligocene–Miocene succession of Denmark. Geol. Surv. Den. Greenl. Bull. 2010, 22, 1–92. [Google Scholar]
- Reid, E.M.; Chandler, M.E.J. Catalogue of Cainozoic Plants in the Department of Geology. Volume 1. The Bembridge Flora; British Museum (Natural History): London, UK, 1926. [Google Scholar]
- Reid, C.; Reid, E.M. Fossil flora of Tegelen-sur-Meuse, near Venloo, in the Province of Limburg. Verh. K. Akad. Wet. Amst. 1907, Deel XIII. N° 6, 1–26. [Google Scholar]
- Reid, C.; Reid, E.M. The Pliocene floras of the Dutch-Prussian border. Meded. Rijksopspor. Delftstoffen 1915, 6, 1–178. [Google Scholar]
- Reid, E.M. Recherches sur quelques graines pliocènes du Pont-du-Gail (Cantal). Bull. Société Géologique Fr. 1920, 20, 48–87. [Google Scholar]
- Riškienė, M.A. Novyj vid roda Najas v mezhlednikovykh otlozhenijakh obnazhenija v Bujvidzhjaj. Liet. Geol. Moksl. Tyrim. Institutas Darb. 1974, 27, 119–122. [Google Scholar]
- Saporta, G. Études sur la végétation du niveau du Sud-Est de la France à l’époque tertiaire. Ann. Sci. Nat. Bot. Ser. V 1873, 17, 5–44. [Google Scholar]
- De Saporta, G. Le Monde des Plantes Avant l’apparition de l’homme; G. Masson: Paris, France, 1879. [Google Scholar]
- Schaarschmidt, F. Präparation und Untersuchung der eozänen Pflanzenfossilien von Messel bei Darmstadt. Cour. Forschungsinstitut Senckenb. 1982, 56, 59–77. [Google Scholar]
- Schimper, W.P. Traité de Paléontologie Végétale, Ou: Flore du Monde Primitif Dans Ses Rapports Avec les Formations Géologique et la Flore du Monde Actuel; Baillière: Paris, France, 1869; Volume 1. [Google Scholar]
- Sille, N.P.; Collinson, M.E.; Kucera, M.; Hooker, J.J. Evolution within the charophyte genus Harrisichara, late Paleogene, southern England; environmental and biostratigraphic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 208, 153–173. [Google Scholar] [CrossRef]
- Sille, N.P.; Collinson, M.E.; Kucera, M.; Hooker, J.J. Morphological evolution of Stratiotes through the Paleogene in England: An example of microevolution in flowering plants. Palaios 2006, 21, 272–288. [Google Scholar] [CrossRef]
- Straus, A. Die oberpliozäne Flora von Willershausen am Harz. Berichte Naturhistorischen Ges. Hann. 1992, 134, 7–115. [Google Scholar]
- Stuchlik, L.; Szynkiewicz, A.; Łańcuka-Środoniowa, M.; Zastawniak, E. Results of the hitherto palaeobotanical investigations of the Tertiary brown coal bed “Bełchatów” (Central Poland). Acta Palaeobot. 1990, 30, 259–305. [Google Scholar]
- Sukaczev, V.N. O vidakh roda Najas iz otlozhenij Likhvinskogo mezhlednikov’ja. Bot. Zhurnal 1958, 43, 239–242. [Google Scholar]
- Ţicleanu, N. Macroflore et Végétation Daciènnes Du Bassin Dacique. In Chronostratigraphie und Neostratotypen. Neogen der Zentrale Paratethys. Bd 9; Marinescu, F.L., Papaianopol, I., Eds.; Rumänische Akademie: Bucharest, Romania, 1995; Volume 11, pp. 473–497. [Google Scholar]
- Tobolski, K. Holocene vegetational development based on the Kluki reference site in the Gardno-Łeba Plain. Acta Palaeobot. 1987, 27, 179–222. [Google Scholar]
- Tolonen, K. The history of the Stratiotes lakes of northern Fennoscandia in the light of stratigraphical studies. Wahlenbergia 1981, 7, 167–177. [Google Scholar]
- Uhl, D. Neue Funde der fossilen Krebsschere Stratiotes kaltennordheimensis (Zenker) Keilhack (Hydrocharitaceae) aus dem Oberoligozän des Westerwaldes (Rheinland-Pfalz, W-Deutschland). Mitt. Pollichia 2017, 98, 41–50. [Google Scholar]
- Uhl, D.; Bruch, A.A.; Traiser, C.; Klotz, S. Palaeoclimate estimates for the Middle Miocene Schrotzburg flora (S Germany): A multi-method approach. Int. J. Earth Sci. 2006, 95, 1071–1085. [Google Scholar] [CrossRef]
- Velitzelos, D.; Bouchal, J.M.; Denk, T. Review of the Cenozoic Floras and Vegetation of Greece. Rev. Palaeobot. Palynol. 2014, 204, 56–117. [Google Scholar] [CrossRef]
- Vernon, R.O. Geology of citrus and levy counties, Florida. Fla. Geol. Surv. Bull. 1951, 33, 95–111. [Google Scholar]
- Wessel, P.; Weber, C.O. Neuer Beitrag zur Tertiärflora der niederrheinischen Braunkohlenformation. Palaeontographica 1855, 4, 111–168. [Google Scholar]
- Weyland, H. Beiträge zur Kenntnis der Rheinischen Tertiärflora: VI. Vierte Ergänzungen und Berichtigungen zur Flora der Blätterkohle und des Polierschiefers von Rott im Siebengebirge. Palaeontogr. Abt. B 1943, 87, 94–136. [Google Scholar]
- Wieliczkiewicz, F. Antropogenovye Semennye Flory Belorussii i Smezhnykh Oblastej; Nauka i Tekhnika: Minsk, Russia, 1973. [Google Scholar]
- Wieliczkiewicz, F. Istorija Plejstotsenovoj Flory Srednej Polosy Vostochno-Evropejskoj Ravniny. In Sovetskaja Paleokarpologija; Goretzky, G.I., Grichuk, V.P., Eds.; Izdatel’stvo “Nauka”: Moscow, Russia, 1979; pp. 76–121. [Google Scholar]
- Wieliczkiewicz, F. Plejstotsenovye Flory Lednikovykh Oblastej Vostochno-Evropejskoj Ravniny; Nauka i Tekhnika: Minsk, Russia, 1982. [Google Scholar]
- Witte, H. Stratiotes aloides L. funnen i Sveriges postglaciala aflagringar. Geol. Föreningen I Stock. Förhandlingar 1905, 27, 432–451. [Google Scholar] [CrossRef]
- Worobiec, G. New fossil floras from Neogene deposits in the Bełchatów Lignite Mine. Acta Palaeobot. Suppl. 2003, 3, 3–133. [Google Scholar]
- Zoller, H. Ein fossiles, wärmzeitliches Vorkommen von Stratiotes aloides L. in der subalpinen Stufe des unteren Misox (Südschweitz). Veröffentlichungen Geobot. Inst. Rübel Zürich 1958, 33, 280–286. [Google Scholar]
- Lim, J.Y.; Huang, H.; Farnsworth, A.; Lunt, D.J.; Baker, W.J.; Morley, R.J.; Kissling, W.D.; Hoorn, C. The Cenozoic history of palms: Global diversification, biogeography and the decline of megathermal forests. Glob. Ecol. Biogeogr. 2022, 31, 425–439. [Google Scholar] [CrossRef]
- Blakey, R.C.; Fielding, C.R.; Frank, T.D.; Isbell, J.L. Gondwana paleogeography from assembly to breakup—A 500 my odyssey. Geol. Soc. Am. Spec. Pap. 2008, 441, 1–28. [Google Scholar]
- Blakey, R.C. Paleogeography of Europe [CD with Geographic Maps and PowerPoint Presentations]; Colorado Plateau Geosystems, Inc.: Phoenix, AZ, USA, 2011. [Google Scholar]
- Rasser, M.W.; Harzhauser, M.; Anistratenko, O.Y.; Anistrenki, V.V.; Bassi, D.; Belak, M.; Berger, J.P.; Bianchini, G.; Čičič, S.; Ćosović, V.; et al. Palaeogene and Neogene. In The Geology of Central Europe Volume 2: Mesozoic and Cenozoic; McCann, T., Ed.; The Geological Society: London, UK, 2008; pp. 1031–1140. [Google Scholar]
- Mai, D.H. Entwicklung der Wasser-und Sumpfpflanzen Gesellschaften Europas von der Kreide bis ins Quartär. Flora 1985, 176, 449–511. [Google Scholar] [CrossRef]
- Büchel, G.N.; Schaal, S.F.K. Chapter 2. The Formation of the Messel Maar. In Messel. An Ancient Greenhouse Ecosystem; Smith, K.T., Schaal, S.F.K., Habersetzer, J., Eds.; Senkenberg: Frankfurt, Germany, 2018; pp. 7–15. [Google Scholar]
- Lenz, O.K.; Wilde, V.; Moshayedi, M.; Mutzl, J.; Hinderer, M. High-resolution regional vegetation analyses for Eocene records on the Sprendlinger Horst in Southwest Germany. Z. Dtsch. Ges. Geowiss. 2023, 174, 613–632. [Google Scholar] [CrossRef]
- Smith, K.T.; Lehmann, T.; Mayr, G.; Micklich, N.; Rabenstein, R.; Wedmann, S. Chapter 13. The Messel Ecosystem. In Messel. An Ancient Greenhouse Ecosystem; Smith, K.T., Schaal, S.F.K., Habersetzer, J., Eds.; Senckenberg: Frankfurt, Germany, 2018; pp. 303–313. [Google Scholar]
- Nyagah, K. Stratigraphy, depositional history and environments of deposition of Cretaceous through Tertiary strata in the Lamu Basin, southeast Kenya and implications for reservoirs for hydrocarbon exploration. Sediment. Geol. 1995, 96, 43–71. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Zachos, J.C.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Mosbrugger, V.; Utescher, T.; Dilcher, D.L. Cenozoic continental climatic evolution of Central Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 14964–14969. [Google Scholar] [CrossRef] [PubMed]
- Grein, M.; Utescher, T.; Wilde, V.; Roth-Nebelsick, A. Reconstruction of the middle Eocene climate of Messel using palaeobotanical data. Neues Jahrb. Geol. Palaontol.-Abh. 2011, 260, 305–318. [Google Scholar] [CrossRef]
- Tütken, T. Isotope compositions (C, O, Sr, Nd) of vertebrate fossils from the Middle Eocene oil shale of Messel, Germany: Implications for their taphonomy and palaeoenvironment. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 416, 92–109. [Google Scholar] [CrossRef]
- Evans, D.; Sagoo, N.; Renema, W.; Cotton, L.J.; Müller, W.; Todd, J.A.; Saraswati, P.K.; Stassen, P.; Ziegler, M.; Pearson, P.N.; et al. Eocene greenhouse climate revealed by coupled clumped isotope-Mg/Ca thermometry. Proc. Natl. Acad. Sci. USA 2018, 115, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, N.; Valdes, P.; Flecker, R.; Gregoire, L.J. The early Eocene equable climate problem: Can perturbations of climate model parameters identify possible solutions? Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2013, 371, 20130123. [Google Scholar] [CrossRef] [PubMed]
- Lenz, O.K.; Wilde, V.; Riegel, W. Recolonization of a Middle Eocene volcanic site: Quantitative palynology of the initial phase of the maar lake of Messel (Germany). Rev. Palaeobot. Palynol. 2007, 145, 217–242. [Google Scholar] [CrossRef]
- Grímsson, F.; Denk, T.; Zetter, R. Pollen, fruits, and leaves of Tetracentron (Trochodendraceae) from the Cainozoic of Iceland and western North America and their palaeobiogeographic implications. Grana 2008, 47, 1–14. [Google Scholar] [CrossRef]
- Stratiotes aloides, L. Available online: https://wu.jacq.org/WU0152794 (accessed on 15 December 2023).
- Zetter, R. Methodik und Bedeutung einer routinemäßig kombinierten lichtmikroskopischen und rasterelektronenmikroskopischen Untersuchung fossiler Mikrofloren. Cour. Forschungsinstitut Senckenb. 1989, 109, 41–50. [Google Scholar]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Illustrated Pollen Terminology, 2nd ed.; Springer Nature: Cham, Switzerland, 2018. [Google Scholar]
- Ulrich, S.; Grímsson, F. The single-grain method: Adding TEM to the equation. Grana 2020, 59, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, R.H.; Thomas, A. Le Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Ludwig, R. Fossile Crocodiliden Aus der Tertiärformation des Mainzer Beckens; Fischer: Stuttgart, Germany, 1877; Volume 3. [Google Scholar]
- Schaal, S.; Ziegler, W. Messel—Ein Schaufenster in der Geschichte der Erde und des Lebens; Verlag Waldemar Kramer: Frankfurt am Main, Germany, 1988. [Google Scholar]
- Gruber, G.; Micklich, N. (Eds.) Messel Treasures of the Eocene; Hessisches Landesmuseum Darmstadt: Darmstadt, Germany, 2007. [Google Scholar]
- Smith, K.; Schaal, S.; Habersetzer, J. (Eds.) Messel—An Ancient Greenhouse Ecosystem; Senckenberg Gesellschaft für Naturforschung: Frankfurt am Main, Germany, 2018. [Google Scholar]
- Mertz, D.F.; Renne, P.R. A numerical age of for the Messel fossil deposit (UNESCO World Heritage Site) derived from 40Ar/39Ar dating on a basaltic rock fragment. Cour. Forsch.-Inst. Senckenb. 2005, 255, 67–75. [Google Scholar]
- Lenz, O.K.; Wilde, V.; Mertz, D.F.; Riegel, W. New palynology-based astronomical and revised 40Ar/39Ar ages for the Eocene maar lake of Messel (Germany). Int. J. Earth Sci. 2015, 104, 873–889. [Google Scholar] [CrossRef]
- Kaboth-Bahr, S.; Schmitt, C.; Bauersachs, T.; Zeeden, C.; Wonik, T.; Schandl, J.; Lenz, O.; Wedmann, S.; Vasiliev, I.; Mulch, A. Improved chronostratigraphy for the Messel Formation (Hesse, Germany) provides insight into early to middle Eocene climate variability. Newsl. Stratigr. 2024. [Google Scholar] [CrossRef]
- Nyaberi, M.D.; Rop, B.K. Petroleum prospects of Lamu Basin, South-Eastern Kenya. J. Geol. Soc. India 2014, 83, 414–422. [Google Scholar] [CrossRef]
- ICS. International Chronostratigraphic Chart V2023/09. Available online: https://stratigraphy.org/ICSchart/ChronostratChart2023-09.pdf (accessed on 10 January 2024).
- Ronquist, F.; Klopfstein, S.; Vilhelmsen, L.; Schulmeister, S.; Murray, D.L.; Rasnitsyn, A.P. A total-evidence approach to dating with fossils, applied to the early radiation of the hymenoptera. Syst. Biol. 2012, 61, 973–999. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), IEEE, New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Barido-Sottani, J.; Aguirre-Fernández, G.; Hopkins, M.J.; Stadler, T.; Warnock, R. Ignoring stratigraphic age uncertainty leads to erroneous estimates of species divergence times under the fossilized birth-death process. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190685. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018; 67, 901–904. [Google Scholar] [CrossRef]
- Matzke, N. BioGeoBEARS: Biogeography with Bayesian (and Likelihood) Evolutionary Analysis with R Scripts. v.1.1.1 2018. Available online: https://github.com/nmatzke/BioGeoBEARS/releases/tag/v1.1.1 (accessed on 20 January 2024).
- Denk, T.; Grimm, G.W.; Grímsson, F.; Zetter, R. Evidence from “Köppen signatures” of fossil plant assemblages for effective heat transport of Gulf Stream to subarctic North Atlantic during Miocene cooling. Biogeosciences 2013, 10, 7927–7942. [Google Scholar] [CrossRef]
- Vieira, M.; Zetter, R.; Grímsson, F.; Denk, T. Niche evolution versus niche conservatism and habitat loss determine persistence and extirpation in late Neogene European Fagaceae. Quat. Sci. Rev. 2023, 300, 107896. [Google Scholar] [CrossRef]
- Grímsson, F.; Grimm, G.W.; Meller, B.; Bouchal, J.M.; Zetter, R. Combined LM and SEM study of the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part IV. Magnoliophyta 2—Fagales to Rosales. Grana 2016, 55, 101–163. [Google Scholar] [CrossRef]
- Bouchal, J.M.; Güner, T.H.; Velitzelos, D.; Velitzelos, E.; Denk, T. Messinian vegetation and climate of the intermontane Florina–Ptolemais–Servia Basin, NW Greece inferred from palaeobotanical data: How well do plant fossils reflect past environments? R. Soc. Open Sci. 2020, 7, 192067. [Google Scholar] [CrossRef] [PubMed]
- Geier, C.; Bouchal, J.M.; Ulrich, S.; Gross, M.; Zetter, R.; Denk, T.; Grímsson, F. Paleovegetation and paleoclimate inferences of the early late Sarmatian palynoflora from the Gleisdorf Fm. at Gratkorn, Styria, Austria. Rev. Palaeobot. Palynol. 2022, 307, 104767. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Westerhold, T.; Marwan, N.; Drury, A.J.; Liebrand, D.; Agnini, C.; Anagnostou, E.; Barnet, J.S.K.; Bohaty, S.M.; De Vleeschouwer, D.; Florindo, F.; et al. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 2020, 369, 1383–1388. [Google Scholar] [CrossRef]
- Grímsson, F.; Zetter, R.; Halbritter, H.; Grimm, G.W. Aponogeton pollen from the Cretaceous and Paleogene of North America and West Greenland: Implications for the origin and palaeobiogeography of the genus. Rev. Palaeobot. Palynol. 2014, 200, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Grímsson, F.; Grimm, G.W.; Zetter, R. Tiny pollen grains: First evidence of Saururaceae from the Late Cretaceous of western North America. PeerJ 2017, 2017, e3434. [Google Scholar] [CrossRef] [PubMed]
- Grímsson, F.; Grimm, G.W.; Potts, A.J.; Zetter, R.; Renner, S.S. A Winteraceae pollen tetrad from the early Paleocene of western Greenland, and the fossil record of Winteraceae in Laurasia and Gondwana. J. Biogeogr. 2018, 45, 567–581. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.R.; Drummond, A.J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Casas-Gallego, M.; Barrón, E. New pollen genera and species from the Oligocene of northern Spain and a systematic, biostratigraphic and biogeographic re-evaluation of coeval taxa. J. Syst. Palaeontol. 2020, 18, 1961–1994. [Google Scholar] [CrossRef]
- Daghlian, C.P. A Review of the Fossil Record of Monocotyledons. Bot. Rev. 1981, 47, 517–555. [Google Scholar] [CrossRef]
- Davey, R.J. The evolution of certain Upper Cretaceous hystrichospheres from South Africa. Palaeontol. Afr. 1969, 12, 25–51. [Google Scholar]
- Davey, R.J.; Williams, G.L. The genus Hystrichosphaeridium and its allies. Bull. Br. Mus. 1966, S3, 53–106. [Google Scholar]
- Dorofeev, P.I. Dekil'ka odnodol'nykh iz neogenovoy flory Ukrayiny. Ukrayins’kyi Bot. Zhurnal 1969, 26, 3–9. [Google Scholar]
- Fritz, A.; Allesch, K. Rasterelektronenmikroskopische Dokumentationen zur Pollen- und Sporenflora ausgewählter Blüten- und Sporenpflanzen. Teil 2: Coniferophytina, Liliopsida, Magnoliopsida. Carinth. II 2006, 196, 171–198. [Google Scholar]
- Halbritter, H. Stratiotes Aloides [online]. Available from PalDat—A Palynological Database. 2016. Available online: https://www.paldat.org/pub/Stratiotes_aloides/302834 (accessed on 21 May 2020).
- Ivanov, D.; Ashraf, A.R.; Utescher, T.; Mosbrugger, V.; Slavomirova, E. Late Miocene vegetation and climate of the Balkan region: Palynology of the Beli Breg Coal Basin sediments. Geol. Carpathica 2007, 58, 367–381. [Google Scholar]
- Knobloch, E. Mikropaleontologický výzkum pannonu a pontu na Moravě a Slovensku. Zemn. Plyn Naft. 1981, 26, 741–757. [Google Scholar]
- Krutzsch, W. Atlas der Mittel- und Jungtertiären Dispersen Sporen- und Pollen-Sowie der Mikroplanktonformen des Nördlichen Mitteleuropas 7; Clivia Mueller: Isernhagen, Germany, 1970. [Google Scholar]
- Stachurska, A.; Sadowska, A.; Dyjor, S. The Neogene flora at Sośnica near Wrocław in the light of geo logical and palynological investigations. Acta Palaeobot. 1973, 14, 147–176. [Google Scholar]
- Stockey, R. The Fossil Record of Basal Monocots. Aliso 2006, 22, 91–106. [Google Scholar] [CrossRef]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Grabowska, I.; Słodkowska, B.; Worobiec, E.; Durska, E. Atlas of Pollen and Spores of the Polish Neogene; W. Szafer Institute of Botany, Polish Academy of Science: Kraków, Poland, 2014. [Google Scholar]
- Rubel, F.; Brugger, K.; Haslinger, K.; Auer, I. The climate of the European Alps: Shift of very high resolution Köppen-Geiger climate zones 1800–2100. Meteorol. Z. 2017, 26, 115–125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).