Modifications in Leaf Anatomical Traits of Coffea spp. Genotypes Induced by Management × Season Interactions
Abstract
1. Introduction
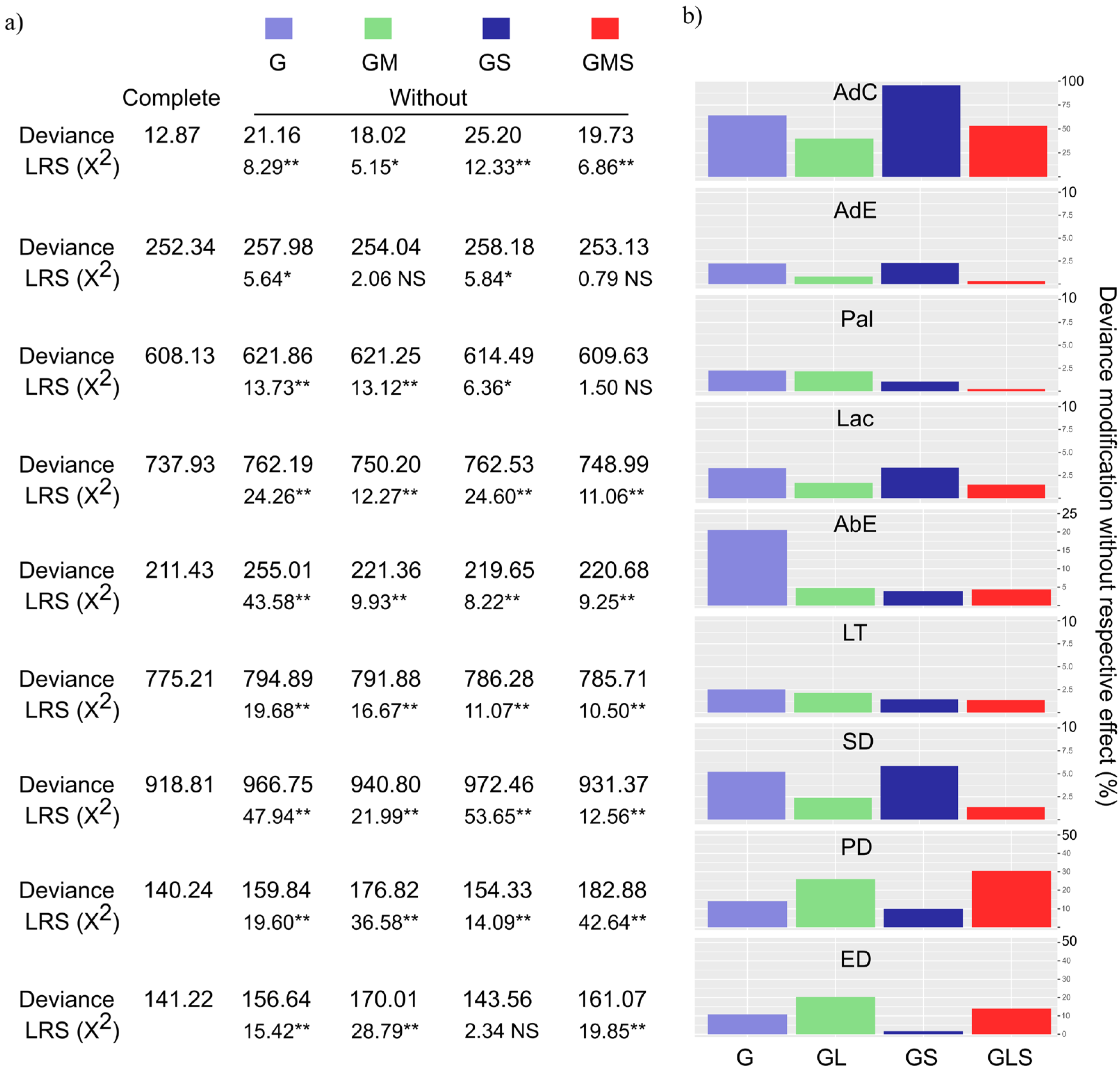
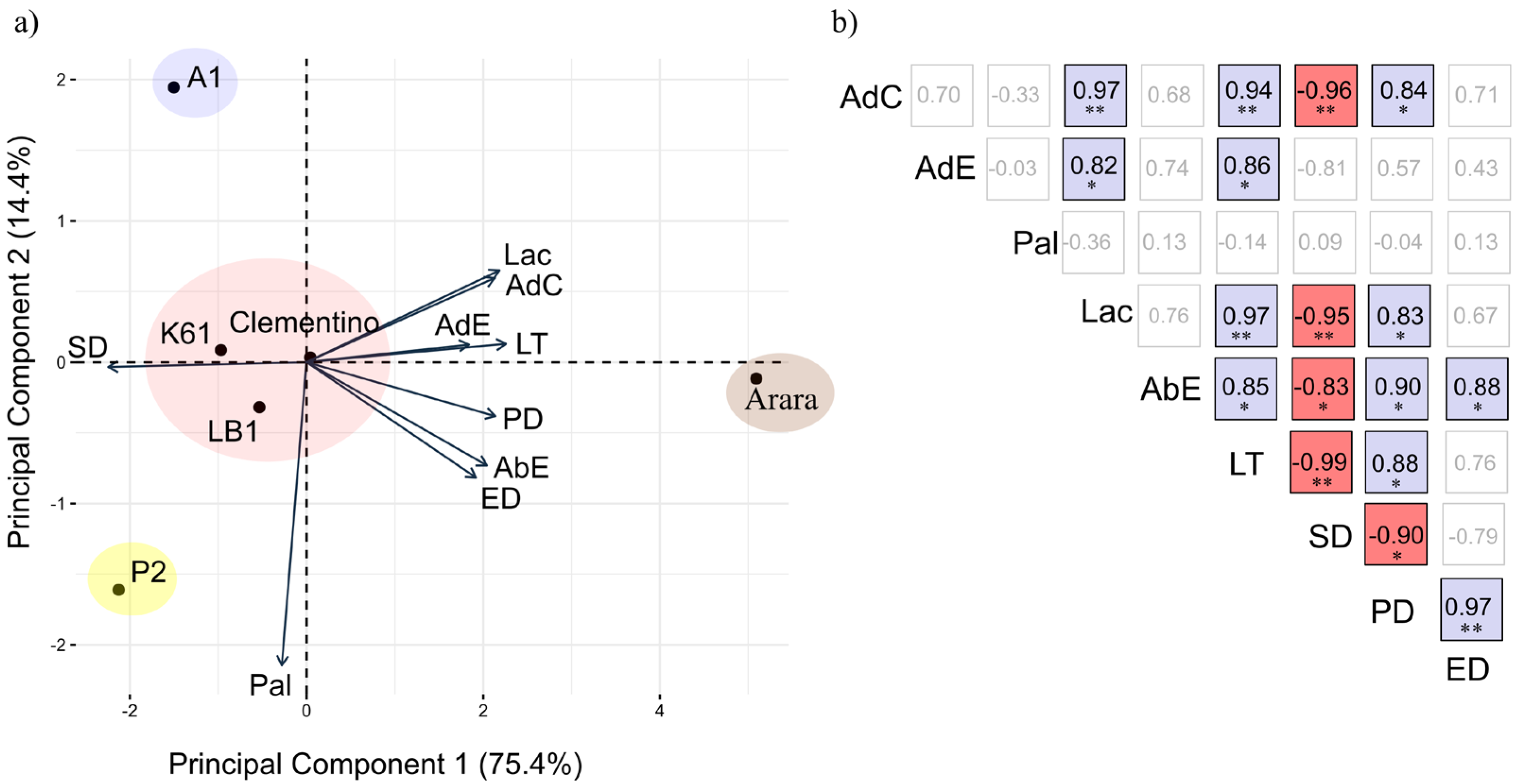
2. Results
Estimation of Genetic Effects and Genotype Classification
3. Discussion
4. Materials and Methods
4.1. Experimental Conditions, Plant Material, and Experimental Design
4.2. Leaf Anatomy
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICO-International Coffee Organization-Monthly Coffee Market Report. 2024. Available online: https://www.icocoffee.org/documents/cy2024-25/cmr-1124-e.pdf (accessed on 12 December 2024).
- Nadaf, S.A.; Shivaprasad, P.; Babou, C.; Hariyappa, N.; Chandrashekar, N.; Kumari, P.; Sowmya, P.R.; Harresh, S.B.; Rajib, P.N.; Nagaraja, J.S.; et al. Coffee (Coffea spp.). In Soil Health Management for Plantation Crops: Recent Advances and New Paradigms; Springer Nature: Singapore, 2024; pp. 337–389. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.C. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Hassan, W.; Nayak, M.A.; Azam, M.F. Intensifying spatially compound heatwaves: Global implications to crop production and human population. Sci. Total Environ. 2024, 932, 172914. [Google Scholar] [CrossRef] [PubMed]
- Bunn, C.; Läderach, P.; Ovalle Rivera, O.; Kirschke, D. A bitter cup: Climate change profile of global production of Arabica and Robusta coffee. Clim. Change 2015, 129, 89–101. [Google Scholar] [CrossRef]
- Lorençone, P.A.; Oliveira Aparecido, L.E.; Lorençone, J.A.; Botega, G.T.; Lima, R.F.; Souza Rolim, G. Climate change and its consequences on the climatic zoning of Coffea canephora in Brazil. Environ. Dev. Sustain. 2023, 26, 8377–8398. [Google Scholar] [CrossRef]
- Piedra-Bonilla, E.B.; Cunha, D.A.; Braga, M.J. Climate variability and crop diversification in Brazil: An ordered probit analysis. J. Clean. Prod. 2020, 256, 120252. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Coffee Production by Region, 1961 to 2022. 2023. Available online: https://ourworldindata.org/grapher/coffee-production-by-region (accessed on 3 March 2025).
- López, M.E.; Santos, I.S.; Oliveira, R.R.; Lima, A.A.; Cardon, C.H.; Chalfun-Junior, A. An overview of the endogenous and environmental factors related to the Coffea arabica flowering process. Beverage Plant Res. 2021, 1, 1–16. [Google Scholar] [CrossRef]
- González-Orozco, C.E.; Porcel, M.; Byrareddy, V.M.; Rahn, E.; Cardona, W.A.; Velandia, D.A.S.; Carrilo, G.A.; Kath, J. Preparing Colombian coffee production for climate change: Integrated spatial modelling to identify potential robusta coffee (Coffea canephora P.) growing areas. Clim. Change 2024, 177, 67. [Google Scholar] [CrossRef]
- Martinez, H.E.; Souza, B.P.; Caixeta, E.T.; Carvalho, F.P.; Clemente, J.M. Water stress changes nitrate uptake and expression of some nitrogen related genes in coffee-plants (Coffea arabica L.). Sci. Hortic. 2020, 267, 109254. [Google Scholar] [CrossRef]
- Santos, C.S.; Freitas, A.; Silva, G.H.B.; Pennacchi, J.P.; Carvalho, M.A.F.; Santos, M.D.O.; Moraes, T.S.J.; Abrahão, J.C.R.; Pereira, A.A.; Carvalho, G.R.; et al. Phenotypic plasticity index as a strategy for selecting water-stress-adapted coffee genotypes. Plants 2023, 12, 4029. [Google Scholar] [CrossRef]
- Silva, L.O.E.; Rodrigues, M.J.L.; Ferreira, M.F.S.; Almeida, R.N.; Ramalho, J.C.; Rakocevic, M.; Partelli, F.L. Modifications in floral morphology of Coffea spp. genotypes at two distinct elevations. Flora 2024, 310, 152443. [Google Scholar] [CrossRef]
- Akpertey, A.; Anim, E.; Adu-Gyamfi, P.K.K.; Dadzie, A.M.; Nyadanu, D.; Ofori, D. Exploring genotype x environment interaction in Robusta coffee for growth and yield stability under tropical environments. J. Crop Sci. Biotechnol. 2023, 26, 179–197. [Google Scholar] [CrossRef]
- Gebreselassie, H.; Tesfaye, B.; Gedebo, A.; Tolessa, K. Genotype by environment interaction and stability analysis using AMMI and GGE-biplot models for yield of Arabica coffee genotypes in south Ethiopia. J. Crop Sci. Biotechnol. 2024, 27, 65–77. [Google Scholar] [CrossRef]
- Souza, C.A.; Rocha, R.B.; Teixeira, A.L.; Alves, E.A.; Espindula, M.C. Genotype-environment interaction for the sensory profile of Coffea arabica lines in high temperature regions in the Western Amazon. Genet. Mol. Res. 2021, 20, gmr18809. [Google Scholar] [CrossRef]
- Araújo, L.F.B.; Espindula, M.C.; Rocha, R.B.; Torres, J.D.; Campanharo, M.; Pego, W.F.O.; Rosa, S.D.S. Genetic divergence based on leaf vegetative and anatomical traits of Coffea canephora clones. Sem. Ciênc. Agrár. 2021, 42, 2717–2734. [Google Scholar] [CrossRef]
- Alberto, N.J.; Ferreira, A.; Ribeiro-Barros, A.I.; Aoyama, E.M.; Silva, L.O.E.; Rakocevic, M.; Partelli, F.L. Plant morphological and leaf anatomical traits in Coffea arabica L. cultivars cropped in Gorongosa Mountain, Mozambique. Horticulturae 2024, 10, 1002. [Google Scholar] [CrossRef]
- Dorken, V.M.; Lepetit, B. Morpho-anatomical and physiological differences between sun and shade leaves in Abies alba Mill. (Pinaceae, Coniferales): A combined approach. Plant Cell Environ. 2018, 41, 1683–1697. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2018, 197, 92–101. [Google Scholar] [CrossRef]
- Kim, G.T.; Yano, S.; Kozuka, T.; Tsukaya, H. Photomorphogenesis of leaves: Shade-avoidance and differentiation of sun and shade leaves. Photochem. Photobiol. Sci. 2005, 4, 770–774. [Google Scholar] [CrossRef]
- Hoshino, R.; Yoshida, Y.; Tsukaya, H. Multiple steps of leaf thickening during sun-leaf formation in Arabidopsis. Plant J. 2019, 100, 738–753. [Google Scholar] [CrossRef]
- Boardman, N.T. Comparative photosynthesis of sun and shade plants. Ann. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Singla, A.; Sharma, R.; Chhabra, R.; Vij, L.; Singh, P. Effect of varying shade intensities of green net on growth and stomatal attributes of different Ocimum species. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 865–878. [Google Scholar] [CrossRef]
- Baroni, D.F.; Souza, G.A.; Bernado, W.D.P.; Santos, A.R.; Barcellos, L.C.D.S.; Barcelos, L.F.; Correia, L.Z.; Almeida, C.M.; Verdim Filho, A.C.; Rodrigues, W.P.; et al. Stomatal and non-stomatal leaf responses during two sequential water stress cycles in young Coffea canephora plants. Stresses 2024, 4, 575–597. [Google Scholar] [CrossRef]
- Avila, R.T.; Cardoso, A.A.; Almeida, W.L.; Costa, L.C.; Machado, K.L.; Barbosa, M.L.; DaMatta, F.M. Coffee plants respond to drought and elevated [CO2] through changes in stomatal function, plant hydraulic conductance, and aquaporin expression. Environ. Exper. Bot. 2020, 177, 104148. [Google Scholar] [CrossRef]
- Caine, R.S.; Harriso, E.L.; Sloan, J.; Flis, P.M.; Fischer, S.; Khan, M.S.; Nguyen, P.T.; Nguyen, L.T.; Gray, J.E.; Croft, H. The influences of stomatal size and density on rice abiotic stress resilience. New Phytol. 2023, 237, 2180–2195. [Google Scholar] [CrossRef]
- Korner, C.; Allison, A.; Hilscher, H. Altitudinal variation of leaf diffusive conductance and leaf anatomy in heliophytes of montane New Guinea and their interrelation with microclimate. Flora 1983, 174, 91–135. [Google Scholar] [CrossRef]
- Hu, J.J.; Xing, Y.W.; Su, T.; Huang, Y.J.; Zhou, Z.K. Stomatal frequency of Quercus glauca from three material sources shows the same inverse response to atmospheric CO2. Ann. Bot. 2019, 123, 1147–1158. [Google Scholar] [CrossRef]
- Wang, R.; Yu, G.; He, N.; Wang, Q.; Xia, F.; Zhao, N.; Xu, Z.; Ge, J.; Li, C. Elevation-related variation in leaf stomatal traits as a function of plant functional type: Evidence from Changbai Mountain, China. PLoS ONE 2014, 9, e115395. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Calixto, E.S.; Iqbal, Z.; Abdalla, M.; Alsamadany, H.; Parvez, R.; Romman, M.; Ali, N.; Sakhi, S.; et al. Species-specific and altitude-related variations in stomatal features of Berberis lycium Royle and B. parkeriana CK Schneid. Bot. Lett. 2022, 169, 43–50. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Li, Q.; Quan, C. Reduced stomatal frequency with rising elevation for Kobresia royleana on the Tibetan Plateau. Glob. Ecol. Conserv. 2020, 24, e01326. [Google Scholar] [CrossRef]
- Carelli, M.L.C.; Queiroz-Voltan, R.B.; Fahl, J.I.; Trivelin, P.C.O. Leaf anatomy and carbon isotope composition in Coffea species related to photosynthetic pathway. Braz. J. Plant Physiol. 2003, 15, 19–24. [Google Scholar] [CrossRef]
- Santos, C.S.D.; Matos, N.M.S.D.; Rezende, T.T.; Mauri, J.; Rodrigues, G.C.; Veiga, A.D.; Bartholo, G.F.; Carvalho, M.A.D.F. Agronomic, anatomic and physiological characterization of Coffea arabica L. genotypes on irrigated system in the Central Cerrado. Coffee Sci. 2022, 17, e172021. [Google Scholar] [CrossRef]
- Partelli, F.L.; Giles, J.A.D.; Oliosi, G.; Covre, A.M.; Ferriera, A.; Rodrigues, V.M. Tributun: A coffee cultivar developed in partnership with farmers. Crop Breed. Appl. Biotechnol. 2020, 20, e30002025. [Google Scholar] [CrossRef]
- Partelli, F.L.; Covre, A.M.; Oliosi, G.; Covre, D.T. Monte Pascoal: First clonal conilon coffee cultivar for southern Bahia-Brazil. Funct. Plant Breed. J. 2021, 3, 107–112. [Google Scholar] [CrossRef]
- Santin, M.R.; Coelho, M.C.; Sayd, R.M.; Peixoto, J.R.; Amabile, R.F. Yield, maturation cycle, and estimates of genetic parameters of Robusta coffee genotypes under irrigation in the Cerrado. Crop Breed. Appl. Biotechnol. 2019, 19, 387–394. [Google Scholar] [CrossRef]
- Ferrão, R.G.; Ferreira, A.; Cruz, C.D.; Cecon, P.R.; Ferrão, M.A.G.; Fonseca, A.F.A.; Carneiro, P.C.S.; Silva, M.F. Inter-trait relations for direct and indirect selection in coffee. Crop Breed. Appl. Biotechnol. 2008, 8, 271–278. [Google Scholar] [CrossRef]
- Partelli, F.L.; Araújo, A.V.; Vieira, H.D.; Dias, J.R.M.; Menezes, L.F.T.D.; Ramalho, J.C. Microclimate and development of ’Conilon’ coffee intercropped with rubber trees. Pesq. Agropec. Bras. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Assis, B.D.P.; Gross, E.; Pereira, N.E.; Mielke, M.S.; Júnior, G.A.G. Growth response of four Conilon coffee varieties (Coffea canephora Pierre ex A. Froehner) to different shading levels. J. Agric. Sci. 2019, 11, 29. [Google Scholar] [CrossRef]
- Silva Neto, F.J.D.; Morinigo, K.P.G.; Guimarães, N.D.F.; Gallo, A.D.S.; Souza, M.D.B.D.; Stolf, R.; Fontanetti, A. Shade trees spatial distribution and its effect on grains and beverage quality of shaded coffee trees. J. Food Qual. 2018, 2018, 7909467. [Google Scholar] [CrossRef]
- Morais, H.; Medri, M.E.; Marur, C.J.; Caramori, P.H.; Ribeiro, A.M.D.A.; Gomes, J.C. Modifications on leaf anatomy of Coffea arabica caused by shade of pigeonpea (Cajanus cajan). Braz. Arch. Biol. Technol. 2004, 47, 863–871. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, U. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef]
- Cruz, C.D.; Carneiro, P.C.S.; Regazzi, A.J. Modelos Biométricos Aplicados ao Melhoramento Genético, 3rd ed.; Editora UFV: Viçosa, Minas Gerais, Brasil, 2014; p. 668. [Google Scholar]
- Camargo, Â.P.D.; Camargo, M.B.P.D. Definição e esquematização das fases fenológicas do cafeeiro arábica nas condições tropicais do Brasil. Bragantia 2011, 60, 65–68. [Google Scholar] [CrossRef]
- Rodrigues, W.P.; Silva, J.R.; Ferreira, L.S.; Machado Filho, J.A.; Figueiredo, F.A.; Ferraz, T.M.; Bernado, W.P.; Bezerra, L.B.S.; Pereira, D.; Cespon, L.; et al. Stomatal and photochemical limitations of photosynthesis in coffee (Coffea spp.) plants subjected to elevated temperatures. Crop Pastu. Sci. 2018, 69, 317–325. [Google Scholar] [CrossRef]
- Pérez-Molina, J.P.; Toledo Picoli, E.A.; Oliveira, L.A.; Silva, B.; Souza, G.A.; Santos Rufino, J.L.; Pereira, A.A.; Ribeiro, M.F.; Malvicini, G.L.; Turello, L.; et al. Treasured exceptions: Association of morphoanatomical leaf traits with cup quality of Coffea arabica L. cv. “Catuaí”. Food Res. Int. 2021, 141, 110118. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.O.E.; Rodrigues, M.J.L.; Almeida, R.N.; Semedo, J.N.; Rakocevic, M.; Partelli, F.L. Towards a minimum number of key flower traits in studies of Coffea spp. phenotype variability. Sci. Hortic. 2024, 337, 113513. [Google Scholar] [CrossRef]
- Poorter, H.; Knopf, O.; Wright, I.J.; Temme, A.A.; Hogewoning, S.W.; Graf, A.; Cernusak, L.A.; Pons, T.L. A meta-analysis of responses of C3 plants to atmospheric CO2: Dose–response curves for 85 traits ranging from the molecular to the whole-plant level. New Phytol. 2022, 233, 1560–1596. [Google Scholar] [CrossRef]
- Kim, E.D.; Torii, K.U. Stomatal cell fate commitment via transcriptional and epigenetic control: Timing is crucial. Plant Cell Environ. 2024, 47, 3288–3298. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Spavore, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Instituto Nacional de Meteorologia-INMET. Dados das Estações Meteorológicas A616–São Mateus, ES, Brasil e A633-Venda Nova do Imigrante, ES, Brasil. Available online: https://mapas.inmet.gov.br/ (accessed on 25 July 2022).
- Iuss Working Group Wrb. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps: Update, 2015; FAO: Rome, Italy, 2025. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System, 5th ed.; Embrapa: Brazil, Brasília, 2018. [Google Scholar]
- Araújo, A.V.; Partelli, F.L.; Oliosi, G.; Pezzopane, J.R.M. Microclimate, development and productivity of robusta coffee shaded by rubber trees and at full sun. Rev. Ciênc. Agron. 2016, 47, 700–709. [Google Scholar] [CrossRef]
- Paye, H.S.; Partelli, F.L.; Martins, A.G.; Siebeneichler, E.A. Recomendação de adubação e calagem. In CAFÉ CONILON: Conhecimento Para Superar Desafios; Partelli, F.L., Espindula, M.C., Eds.; CAUFES: Alegre, Brazil, 2019; pp. 75–98. [Google Scholar]
- Klein, D.E.; Moreira, G.V.; Silva-Neto, S.J.; Da Cunha, M. The structure of colleters in several species of Simira (Rubiaceae). Ann. Bot. 2004, 94, 733–740. [Google Scholar] [CrossRef]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal; EDUR: Seropédica, Brazil, 1997. [Google Scholar]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Resende, M.D.V. Software Selegen-REML/BLUP: A useful tool for plant breeding. Crop Breed. Appl. Biotechnol. 2016, 16, 330–339. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 18 December 2024).



| Traits | h2 ± sd | MLAw | MHAw | AFSw | MLAs | MHAs | AFSs | Overall |
|---|---|---|---|---|---|---|---|---|
| AdC | 0.20 ± 0.12 | 3.75 | 3.61 | 3.39 | 3.42 | 4.50 | 4.40 | 3.84 |
| AdE | 0.01 ± 0.02 | 19.39 | 21.08 | 23.34 | 18.93 | 23.84 | 24.71 | 21.88 |
| Pal | 0.10 ± 0.01 | 50.91 | 70.21 | 25.46 | 87.23 | 76.55 | 32.97 | 57.22 |
| Lac | 0.21 ± 0.11 | 171.94 | 182.84 | 144.03 | 183.91 | 162.75 | 142.68 | 164.69 |
| AbE | 0.12 ± 0.09 | 17.44 | 17.31 | 20.07 | 15.83 | 18.42 | 21.73 | 18.47 |
| LT | 0.10 ± 0.08 | 263.42 | 295.04 | 216.28 | 309.33 | 286.06 | 226.49 | 266.10 |
| SD | 0.37 ± 0.15 | 374.01 | 319.29 | 161.56 | 302.79 | 334.56 | 165.07 | 276.21 |
| PD | 0.75 ± 0.22 | 19.51 | 18.80 | 19.09 | 19.17 | 18.86 | 17.32 | 18.79 |
| ED | 0.31 ± 0.14 | 13.20 | 11.90 | 12.04 | 11.00 | 11.61 | 9.00 | 11.46 |
| Genotype | Pal | LT | SD | |||
|---|---|---|---|---|---|---|
| Intercept | Stability | Intercept | Stability | Intercept | Stability | |
| Clementino | −3.10 | 1.06 | −14.32 | 1.06 | 18.68 | 0.94 |
| A1 | 6.20 | 0.79 | 29.64 | 0.85 | 52.12 | 0.92 |
| P2 | −7.12 | 1.21 | −38.65 | 1.10 | −87.24 | 1.49 |
| LB1 | −3.95 | 1.11 | −51.16 | 1.18 | −40.23 | 1.11 |
| K61 | −0.90 | 0.99 | −18.51 | 1.03 | 50.67 | 0.89 |
| Arara | 8.99 | 0.82 | 93.00 | 0.78 | 5.99 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.O.E.; Almeida, R.N.d.; Feitoza, R.B.B.; Da Cunha, M.; Partelli, F.L. Modifications in Leaf Anatomical Traits of Coffea spp. Genotypes Induced by Management × Season Interactions. Plants 2025, 14, 828. https://doi.org/10.3390/plants14050828
Silva LOE, Almeida RNd, Feitoza RBB, Da Cunha M, Partelli FL. Modifications in Leaf Anatomical Traits of Coffea spp. Genotypes Induced by Management × Season Interactions. Plants. 2025; 14(5):828. https://doi.org/10.3390/plants14050828
Chicago/Turabian StyleSilva, Larícia Olária Emerick, Rafael Nunes de Almeida, Rodrigo Barbosa Braga Feitoza, Maura Da Cunha, and Fábio Luiz Partelli. 2025. "Modifications in Leaf Anatomical Traits of Coffea spp. Genotypes Induced by Management × Season Interactions" Plants 14, no. 5: 828. https://doi.org/10.3390/plants14050828
APA StyleSilva, L. O. E., Almeida, R. N. d., Feitoza, R. B. B., Da Cunha, M., & Partelli, F. L. (2025). Modifications in Leaf Anatomical Traits of Coffea spp. Genotypes Induced by Management × Season Interactions. Plants, 14(5), 828. https://doi.org/10.3390/plants14050828








