Natural Products for Melanoma Therapy: From Traditional Medicine to Modern Drug Discovery
Abstract
:1. Introduction
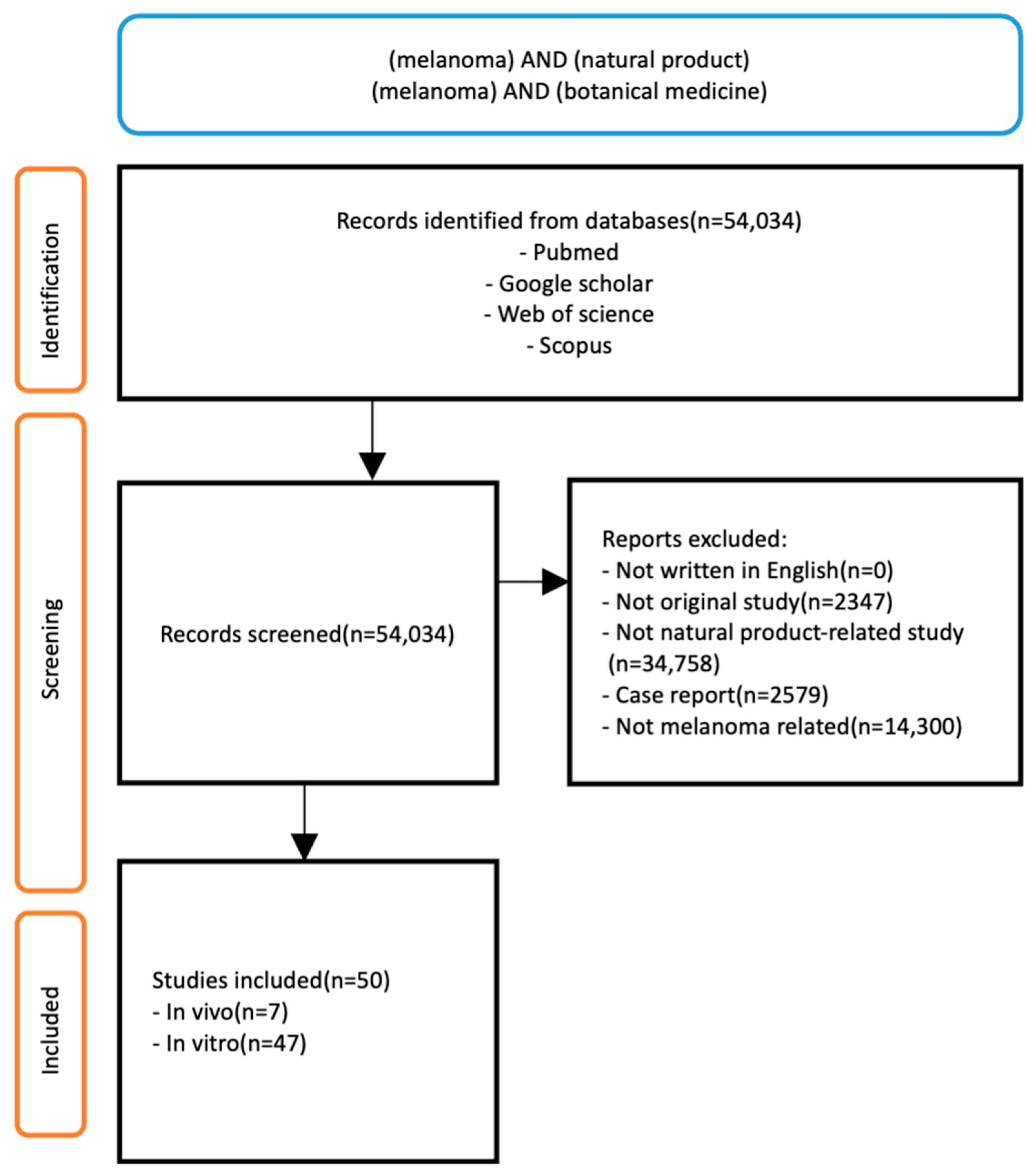
2. Methods
2.1. Search Strategy
2.2. Study Selection Criteria
2.3. Data Extraction
3. Anti-Melanoma Effect of Natural Products
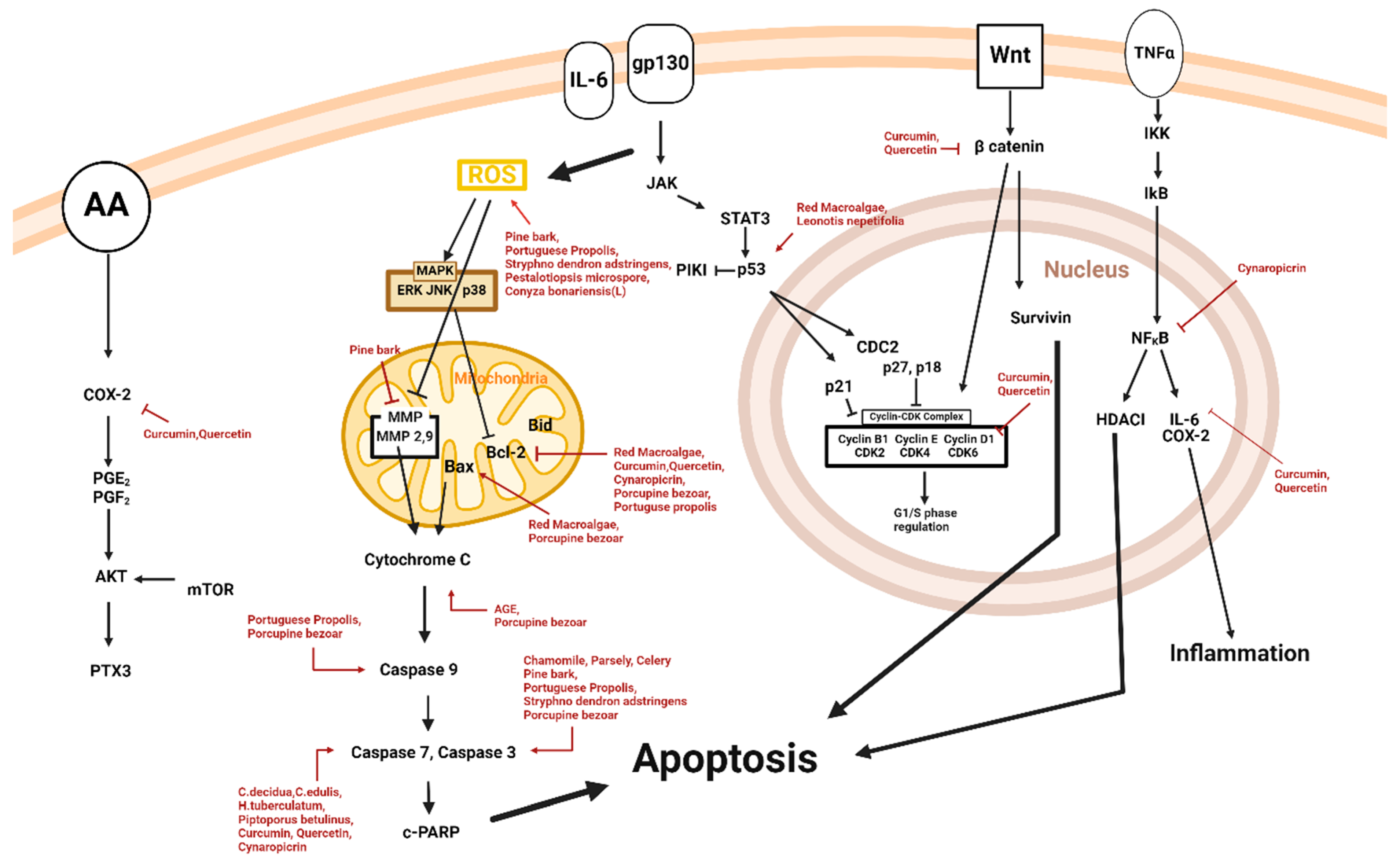
3.1. Apoptosis and Natural Products
| Classification | Drug | Source | Cell Line/Animal Model | Dose/Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Animal | Porcupine bezoar | Hystrix Brachyura | A375 | 1, 2, 3, 4 µg/mL; 24, 48, 72, 96 h | Induction of apoptotic activity | ↑Bax, cyt-c, c-caspase-3, c-caspase-9 ↓Bcl-2 | [18] |
| Diarylheptanoid, Flavonoid | Curcumin, quercetin | Curcuma longa L. | A375 | 1.5, 3.1, 6.3, 12.5, 25, 50 μM; 24 h | Induction of apoptosis | ↓DVL2, β-catenin, cyclin D1, Cox2, Axin2, Bcl-2 ↑c-caspase-3, c-caspase-7 | [19] |
| Fungi | Pestalotiopsis microspora | Gymnema sylvestre leaves | B16F10 | 10, 20, 30, 40, 50, 100, 150, 200 μg/mL | Induction of apoptosis | ↑ROS | [20] |
| Plant Extract | Aged garlic | Allium sativum L. | M14 WT | 1, 5, 7, 10 mg/mL; 24, 48, 72 h | Initiation of intrinsic apoptosis | ↑Cyt c, Smac, Diablo | [21] |
| Plant Extract | Cashew gum | Anacardium occidentale Linn | B16F10 | 3.125, 6.25, 12.5, 25, 50, 100 μg/mL; 72 h | Induction of cell death and apoptosis | ↓γH2AX | [22] |
| C57BL/6 mice | 50, 100 mg/kg; 14 d | ||||||
| Plant Extract | C. decidua, C. edulis H. tuberculatum, | C. decidua, C. edulis H. tuberculatum | A375, B16F10 | 0.45, 10, 15 µg/mL; 48 h | Induction of apoptosis | ↑c-caspase-3, c-caspase-7 | [23] |
| Plant Extract | Chamomile, parsley, celery | Matricaria chamomilla L., Petroselinum crispum (Mill.) Nym. ex A. W. Hill, Apium graveolens L. | A375 | 10, 30, 60 μg/mL; 72 h | Induction of apoptotic activity | ↑c-caspase-3 ↓ IL-10 | [24] |
| Plant Extract | Conyza bonariensis (L.) | Conyza bonariensis (L.) | SK-MEL-28 | 20 μg/mL; 48 h | Induction of apoptosis | ↑ROS | [25] |
| Plant Extract | Curcumin, demethoxycurcumin, bisdemethoxycurcumin | Curcuma longa Linn | A2058 | 5, 10, 15, 20, 25 μΜ; 24, 48 h | Initiation of apoptosis | [26] | |
| Plant Extract | Curzerene | Eugenia uniflora | HCT-116, AGP-01, SKMEL-19, MRC-5 | 5, 10 μM; 72 h | Induction of apoptosis | [27] | |
| Plant Extract | Hibiscus, cinnamomum | Hibiscus syriacus, Cinnamomum loureirii Nees | G361 | 62.5, 125, 250, 500, 1000 μg/mL; 48 h | Induction of apoptosis | [28] | |
| Plant Extract | Leonotis nepetifolia | Leonotis nepetifolia | A375 | 500 µg/mL; 24 h | Induction of apoptosis | ↑p53 | [29] |
| Plant Extract | Pine bark | Pinus maritima | A375 | 5, 25, 50, 100 µg/mL; 12, 24, 48 h | Induction of apoptosis | ↑ROS, c-caspase-3 ↓MMP-9 | [37] |
| Plant Extract | Piptoporus betulinus | Piptoporus betulinus | Hs27, A375, WM115 | 2.5, 5, 10 μL/mL; 24 h | Induction of apoptosis | ↑c-caspase-3, c-caspase-7 | [31] |
| Plant Extract | Portuguese propolis | Apis mellifera L. | A375, WM9 | 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60 µg/mL; 72 h | Activation of apoptosis | ↑ROS, Bax, c-caspase-3, c-caspase-9, p53 ↓Bcl-XL, Bcl-2 | [32] |
| Plant Extract | Red macroalgae | Gelidium latifolium | B16F10 | 10, 50, 100, 200 μg/mL; 72 h | Induction of apoptosis | ↑p53, Bax, Bak ↓Bcl-2 | [33] |
| Plant Extract | Rhamnus alaternus | Rhamnus alaternus | B16F10 | 10, 20, 40 µg/mL; 48 h | Induction of apoptosis | [34] | |
| Plant Extract | Stryphnodendron adstringens | Stryphnodendron adstringens | B16F10 | 10, 25, 50, 125, 250, 500 µg/mL; 24, 48 h | Promotion of apoptosis | ↑ROS, c-caspase-3 | [35] |
| Plant Extract | Thai water lily | Nymphaea stellate | B16F10 | 200, 400, 600 800, 1000 μg/mL; 24 h | Induction of apoptosis | [36] | |
| Terpenoid | Cynaropicrin | Centaurea drabifolia subsp.detonsa | A375 | 30 μM; 24, 48 h | Induction of apoptosis | ↑c-caspase-3, c-caspase-7 ↓Bcl-2, NF-KB | [30] |
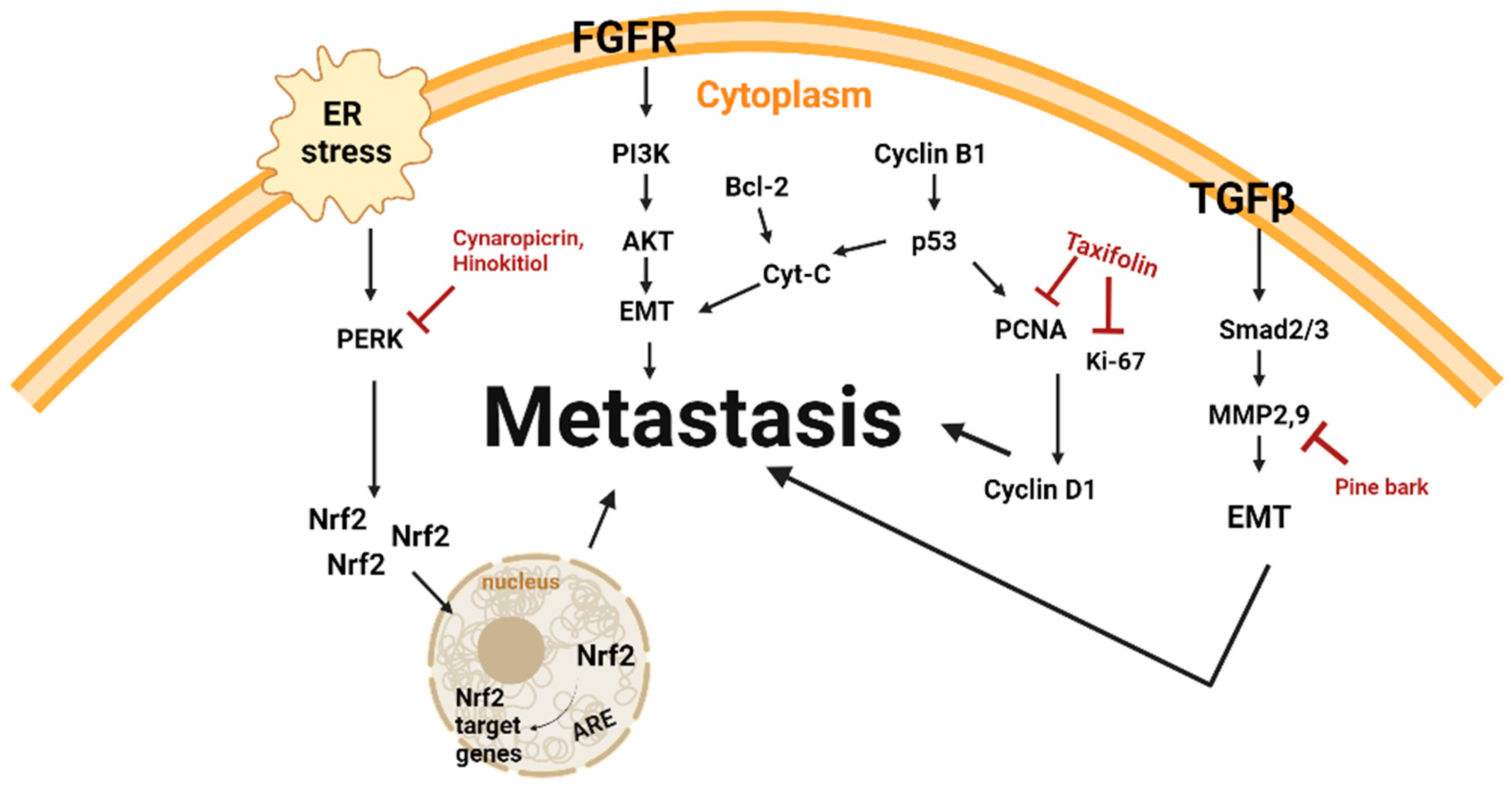
3.2. Anti-Metastasis and Natural Products
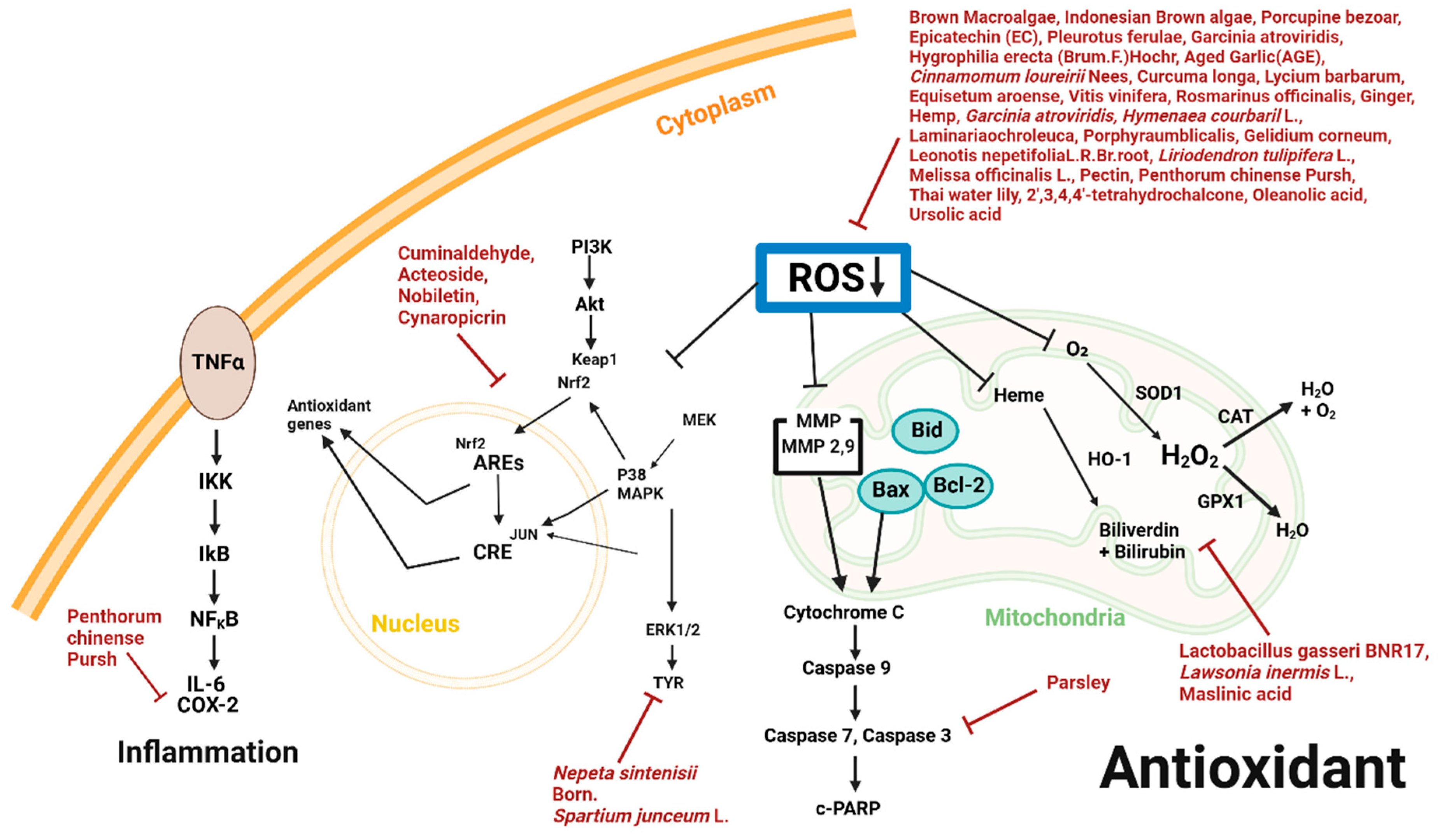
3.3. Antioxidant and Natural Products
3.4. Anti-Angiogenesis and Natural Product
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- PDQ Adult Treatment Editorial Board. Melanoma Treatment (PDQ®)—Patient Version; National Cancer Institute: Bethesda, MD, USA, 2023. [Google Scholar]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Melanoma of Skin. In Cancer Today; Global Cancer Observatory: Lyon, France, 2024. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Estimated Number of New Cases from 2022 to 2025, Both Sexes, Age [0–85+]. Globocan 2022. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?years=2025&single_unit=10000&cancers=16 (accessed on 3 January 2025).
- Osman, A.; Nigro, A.R.; Brauer, S.F.; Borda, L.J.; Roberts, A.A. Epidemiology and primary location of melanoma in Asian patients: A surveillance, epidemiology, and end result-based study. JAAD Int. 2024, 16, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Maretha, D.E.; Afriansyah, D.; Wati, D.S.; Masri, M.; Dwiyanti, A.R.; Hanif, M.I.; Wardoyo, S. Test comparison of seeds and skins extract of duku’s fruit (Lansium domesticum Corr.) against the amount of melanin pigment of skin mencit (Mus musculus) to prevent premature aging of the skin. Adv. Tradit. Med. 2022, 22, 875–883. [Google Scholar] [CrossRef]
- Petrie, T.; Samatham, R.; Witkowski, A.M.; Esteva, A.; Leachman, S.A. Melanoma Early Detection: Big Data, Bigger Picture. J. Investig. Dermatol. 2019, 139, 25–30. [Google Scholar] [CrossRef]
- Jeong, M.; Cho, J.; Lim, D.; Choi, M.; Park, Y.; Cheong, Y.; Kang, Y.; Kang, I.; Kim, S.; Kim, D. The biological effects of Rosa rugosa extract on keratinocyte differentiation and enhancement of skin barrier function. Adv. Tradit. Med. 2024. [Google Scholar] [CrossRef]
- Morgese, F.; Sampaolesi, C.; Torniai, M.; Conti, A.; Ranallo, N.; Giacchetti, A.; Serresi, S.; Onofri, A.; Burattini, M.; Ricotti, G.; et al. Gender Differences and Outcomes in Melanoma Patients. Oncol. Ther. 2020, 8, 103–114. [Google Scholar] [CrossRef]
- Ragab, M.; Choudhry, H.; Al-Rabia, M.W.; Binyamin, S.S.; Aldarmahi, A.A.; Mansour, R.F. Early and accurate detection of melanoma skin cancer using hybrid level set approach. Front. Physiol. 2022, 13, 965630. [Google Scholar] [CrossRef]
- Treating Melanoma Skin Cancer. Available online: https://www.cancer.org/cancer/types/melanoma-skin-cancer/treating.html (accessed on 3 January 2025).
- Kahlon, N.; Doddi, S.; Yousif, R.; Najib, S.; Sheikh, T.; Abuhelwa, Z.; Burmeister, C.; Hamouda, D.M. Melanoma Treatments and Mortality Rate Trends in the US, 1975 to 2019. JAMA Netw Open 2022, 5, e2245269. [Google Scholar] [CrossRef]
- Berthenet, K.; Weber, K.; Ichim, G. Sometimes even apoptosis fails: Implications for cancer. Mol. Cell. Oncol. 2020, 7, 1797430. [Google Scholar] [CrossRef]
- Al-Qatati, A.; Aliwaini, S. Combined pitavastatin and dacarbazine treatment activates apoptosis and autophagy resulting in synergistic cytotoxicity in melanoma cells. Oncol. Lett. 2017, 14, 7993–7999. [Google Scholar] [CrossRef]
- Lee, D.-h.; Seong, S.; Won, Y. Traditional Korean medicine as second-line treatment of metastatic colorectal cancer: A case report. Med. Case Rep. Study Protoc. 2021, 2, e0042. [Google Scholar] [CrossRef]
- Farida, S.; Jenie, R.I.; Fakhrudin, N. Calophyllum inophyllum: A Comprehensive Analysis of its Ethnobotanical, Phytochemical, and Pharmacological Properties. Maj. Obat Tradis. 2024, 29, 121–142. [Google Scholar] [CrossRef]
- Schröder, S.; Lee, S.; Efferth, T.; Motoo, Y. Acupuncture and herbal medicine for cancer patients. Evid. Based Complement. Altern. Med. 2013, 2013, 313751. [Google Scholar] [CrossRef] [PubMed]
- Firus Khan, A.Y.; Abdullah Asuhaimi, F.; Jalal, T.K.; Roheem, F.O.; Natto, H.A.; Johan, M.F.; Ahmed, Q.U.; Abdul Wahab, R. Hystrix brachyura Bezoar Characterization, Antioxidant Activity Screening, and Anticancer Activity on Melanoma Cells (A375): A Preliminary Study. Antioxidants 2019, 8, 39. [Google Scholar] [CrossRef]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef]
- Netala, V.R.; Bethu, M.S.; Pushpalatha, B.; Baki, V.B.; Aishwarya, S.; Rao, J.V.; Tartte, V. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 2016, 11, 5683–5696. [Google Scholar] [CrossRef]
- Ohkubo, S.; Dalla Via, L.; Grancara, S.; Kanamori, Y.; García-Argáez, A.N.; Canettieri, G.; Arcari, P.; Toninello, A.; Agostinelli, E. The antioxidant, aged garlic extract, exerts cytotoxic effects on wild-type and multidrug-resistant human cancer cells by altering mitochondrial permeability. Int. J. Oncol. 2018, 53, 1257–1268. [Google Scholar] [CrossRef]
- Barros, A.B.; Moura, A.F.; Silva, D.A.; Oliveira, T.M.; Barreto, F.S.; Ribeiro, W.L.C.; Alves, A.; Araújo, A.J.; Moraes Filho, M.O.; Iles, B.; et al. Evaluation of antitumor potential of cashew gum extracted from Anacardium occidentale Linn. Int. J. Biol. Macromol. 2020, 154, 319–328. [Google Scholar] [CrossRef]
- AlQathama, A.; Bader, A.; Al-Rehaily, A.; Gibbons, S.; Prieto, J.M. In vitro cytotoxic activities of selected Saudi medicinal plants against human malignant melanoma cells (A375) and the isolation of their active principles. Eur. J. Integr. Med. 2022, 49, 102083. [Google Scholar] [CrossRef]
- Danciu, C.; Zupko, I.; Bor, A.; Schwiebs, A.; Radeke, H.; Hancianu, M.; Cioanca, O.; Alexa, E.; Oprean, C.; Bojin, F.; et al. Botanical Therapeutics: Phytochemical Screening and Biological Assessment of Chamomile, Parsley and Celery Extracts against A375 Human Melanoma and Dendritic Cells. Int. J. Mol. Sci. 2018, 19, 3624. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Duarte, S.S.; de Sousa, V.M.; de Souza, R.R.M.; Marques, K.K.G.; de Abrantes, R.A.; do Nascimento, Y.M.; de Sousa, N.F.; Scotti, M.T.; Scotti, L.; et al. The Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae) Exerts an In Vitro Antimelanoma Effect by Inducing Apoptosis and Modulating the MAPKs, NF-κB, and PKB/AKT Signaling Pathways. Pharmaceuticals 2023, 16, 1553. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lu, H.F.; Chen, Y.H.; Chen, J.C.; Chou, W.H.; Huang, H.C. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin induced caspase-dependent and -independent apoptosis via Smad or Akt signaling pathways in HOS cells. BMC Complement. Med. Ther. 2020, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Oh, H.; Lim, Y.; Kumar Kaushik, N.; Nguyen, L.N.; Choi, E.H.; Kim, J.H. Screening of Hibiscus and Cinnamomum Plants and Identification of Major Phytometabolites in Potential Plant Extracts Responsible for Apoptosis Induction in Skin Melanoma and Lung Adenocarcinoma Cells. Front. Bioeng. Biotechnol. 2021, 9, 779393. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Śliwiński, T.; Zajdel, K.; Malinowska, K.; Zielińska-Bliźniewska, H.; Kucharska, E.; Zajdel, R. In vitro and In silico Studies on Leonotis nepetifolia (L.) R. Br. Root Extract against Cancer Cells. Curr. Pharm. Biotechnol. 2022, 23, 1383–1395. [Google Scholar] [CrossRef]
- De Cicco, P.; Busà, R.; Ercolano, G.; Formisano, C.; Allegra, M.; Taglialatela-Scafati, O.; Ianaro, A. Inhibitory effects of cynaropicrin on human melanoma progression by targeting MAPK, NF-κB, and Nrf-2 signaling pathways in vitro. Phytother. Res. 2021, 35, 1432–1442. [Google Scholar] [CrossRef]
- Bożek, J.; Tomala, J.; Wójcik, S.; Kamińska, B.; Brand, I.; Pocheć, E.; Szostak, E. Effects of Piptoporus betulinus Ethanolic Extract on the Proliferation and Viability of Melanoma Cells and Models of Their Cell Membranes. Int. J. Mol. Sci. 2022, 23, 13907. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Celeiro, S.P.; Barbosa-Matos, C.; Freitas, A.S.; Cardoso, S.M.; Viana-Pereira, M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese Propolis Antitumoral Activity in Melanoma Involves ROS Production and Induction of Apoptosis. Molecules 2022, 27, 3533. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Ardiana, N.; Padmi, H.; Ilhami, B.T.K.; Martyasari, N.W.R.; Sunarwidhi, A.L.; Nikmatullah, A.; Widyastuti, S.; Sunarpi, H.; Frediansyah, A. The Antiproliferative and Apoptosis-Inducing Effects of the Red Macroalgae Gelidium latifolium Extract against Melanoma Cells. Molecules 2021, 26, 6568. [Google Scholar] [CrossRef]
- Bouhlel Chatti, I.; Ben Toumia, I.; Krichen, Y.; Maatouk, M.; Chekir Ghedira, L.; Krifa, M. Assessment of Rhamnus alaternus Leaves Extract: Phytochemical Characterization and Antimelanoma Activity. J. Med. Food 2022, 25, 910–917. [Google Scholar] [CrossRef]
- Baldivia, D.D.S.; Leite, D.F.; Castro, D.T.H.; Campos, J.F.; Santos, U.P.D.; Paredes-Gamero, E.J.; Carollo, C.A.; Silva, D.B.; de Picoli Souza, K.; Dos Santos, E.L. Evaluation of In Vitro Antioxidant and Anticancer Properties of the Aqueous Extract from the Stem Bark of Stryphnodendron adstringens. Int. J. Mol. Sci. 2018, 19, 2432. [Google Scholar] [CrossRef]
- Aimvijarn, P.; Palipoch, S.; Okada, S.; Suwannalert, P. Thai Water Lily Extract Induces B16 Melanoma Cell Apoptosis and Inhibits Cellular Invasion Through the Role of Cellular Oxidants. Asian Pac. J. Cancer Prev. 2018, 19, 149–153. [Google Scholar] [PubMed]
- Thaichinda, S.; Tancharoen, S.; Kanekura, T.; Higashi, Y.; Dararat, P.; Kikuchi, K.; Nararatwanchai, T. Pinus maritima Extract Induces Apoptosis in Human Malignant Melanoma Cells via ROS/Caspase-3 Signaling. Nat. Product. Commun. 2020, 15, 1934578X20926889. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Zhang, S.; Yang, J.; Zhu, A.; Sun, J.; Kalvakolanu, D.V.; Cong, X.; Zhang, J.; Tang, J.; et al. Taxifolin inhibits melanoma proliferation/migration impeding USP18/Rac1/JNK/β-catenin oncogenic signaling. Phytomedicine 2024, 123, 155199. [Google Scholar] [CrossRef]
- Silva, R.; Alves, C.P.; Barbosa, F.C.; Santos, H.H.; Adão, K.M.; Granero, F.O.; Figueiredo, C.C.M.; Figueiredo, C.R.; Nicolau-Junior, N.; Silva, L.P. Antioxidant, antitumoral, antimetastatic effect and inhibition of collagenase enzyme activity of Eleutherine bulbosa (Dayak onion) extract: In vitro, in vivo and in silico approaches. J. Ethnopharmacol. 2024, 318 Pt B, 117005. [Google Scholar] [CrossRef]
- Wu, Y.J.; Hsu, W.J.; Wu, L.H.; Liou, H.P.; Pangilinan, C.R.; Tyan, Y.C.; Lee, C.H. Hinokitiol reduces tumor metastasis by inhibiting heparanase via extracellular signal-regulated kinase and protein kinase B pathway. Int. J. Med. Sci. 2020, 17, 403–413. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Padmi, H.; Ilhami, B.T.K.; Martyasari, N.W.R.; Sunarwidhi, A.L.; Widyastuti, S.; Khairinisa, M.A.; Cokrowati, N.; Simangunsong, E.E.; Frediansyah, A. Brown Macroalgae Sargassum cristaefolium Extract Inhibits Melanin Production and Cellular Oxygen Stress in B16F10 Melanoma Cells. Molecules 2022, 27, 8585. [Google Scholar] [CrossRef]
- Sunarwidhi, A.L.; Hernawan, A.; Frediansyah, A.; Widyastuti, S.; Martyasari, N.W.R.; Abidin, A.S.; Padmi, H.; Handayani, E.; Utami, N.W.P.; Maulana, F.A.; et al. Multivariate Analysis Revealed Ultrasonic-Assisted Extraction Improves Anti-Melanoma Activity of Non-Flavonoid Compounds in Indonesian Brown Algae Ethanol Extract. Molecules 2022, 27, 7509. [Google Scholar] [CrossRef]
- Meng, Z.; Oh, S. Antioxidant and Antimelanogenic Activities of Kimchi-Derived Limosilactobacillus fermentum JNU532 in B16F10 Melanoma Cells. J. Microbiol. Biotechnol. 2021, 31, 990–998. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Park, S.M.; Kim, B.; Lee, H.M. Chemical Composition, Antioxidant and Anti-melanogenic Activities of Essential Oils from Chrysanthemum boreale Makino at Different Harvesting Stages. Chem. Biodivers. 2018, 15, e1700506. [Google Scholar] [CrossRef]
- Mustapha, N.; Mokdad-Bzéouich, I.; Maatouk, M.; Ghedira, K.; Hennebelle, T.; Chekir-Ghedira, L. Antitumoral, antioxidant, and antimelanogenesis potencies of Hawthorn, a potential natural agent in the treatment of melanoma. Melanoma Res. 2016, 26, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Sinamabela, J.R.; Rosamah, E.; Kusuma, I.W.; Kuspradini, H.; Alam, A.E.; Amen, Y.; Tanaka, H.; Satria, D.; Shimizu, K.; et al. Antioxidant and Antimelanogenesis Activities of Glyasperin A From Macaranga pruinosa Leaves. Nat. Product. Commun. 2019, 14, 1934578X19867192. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, C.; Zhou, F.; Luo, X.; Li, J.; Zhao, J.; He, J.; Li, X.; Li, J. Chemical composition, antioxidant and antitumor activities of sub-fractions of wild and cultivated Pleurotus ferulae ethanol extracts. PeerJ 2018, 6, e6097. [Google Scholar] [CrossRef]
- Cheimonidi, C.; Samara, P.; Polychronopoulos, P.; Tsakiri, E.N.; Nikou, T.; Myrianthopoulos, V.; Sakellaropoulos, T.; Zoumpourlis, V.; Mikros, E.; Papassideri, I.; et al. Selective cytotoxicity of the herbal substance acteoside against tumor cells and its mechanistic insights. Redox Biol. 2018, 16, 169–178. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.E.; Choi, Y.J.; Jin, Y.J.; Roh, Y.J.; Seol, A.Y.; Song, H.J.; Park, S.H.; Uddin, M.S.; Lee, S.W.; et al. Antioxidative Role of Hygrophila erecta (Brum. F.) Hochr. on UV-Induced Photoaging of Dermal Fibroblasts and Melanoma Cells. Antioxidants 2022, 11, 1317. [Google Scholar] [CrossRef]
- Žitek, T.; Dariš, B.; Finšgar, M.; Knez, Ž.; Bjelić, D.; Knez Hrnčič, M. The Effect of Polyphenolics in Extracts from Natural Materials on Metabolic Activity of Metastatic Melanoma WM-266-4 Cells. Appl. Sci. 2020, 10, 3499. [Google Scholar] [CrossRef]
- Žitek, T.; Leitgeb, M.; Golle, A.; Dariš, B.; Knez, Ž.; Knez Hrnčič, M. The Influence of Hemp Extract in Combination with Ginger on the Metabolic Activity of Metastatic Cells and Microorganisms. Molecules 2020, 25, 4992. [Google Scholar] [CrossRef]
- Chatatikun, M.; Supjaroen, P.; Promlat, P.; Chantarangkul, C.; Waranuntakul, S.; Nawarat, J.; Tangpong, J.; Chiabchalard, A. Antioxidant and Tyrosinase Inhibitory Properties of an Aqueous Extract of Garcinia atroviridis Griff. ex. T. Anderson Fruit Pericarps. Polym. J. 2020, 12, 71–78. [Google Scholar] [CrossRef]
- Spera, K.D.; Figueiredo, P.A.; Santos, P.C.E.; Barbosa, F.C.; Alves, C.P.; Dokkedal, A.L.; Saldanha, L.L.; Silva, L.P.; Figueiredo, C.R.; Ferreira, P.C.; et al. Genotoxicity, anti-melanoma and antioxidant activities of Hymenaea courbaril L. seed extract. An. Acad. Bras. Cienc. 2019, 91, e20180446. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Casas Arrojo, V.; Arrojo Agudo, M.A.; Cárdenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef]
- Raja, W.; Pandey, S. Antitumoral effect of lawsonia inermis extract on melanoma tumor-bearing C57BL/6 mice. Pharmacogn. Mag. 2020, 16, 435. [Google Scholar]
- Quassinti, L.; Maggi, F.; Ortolani, F.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Miano, A.; Bramucci, M. Exploring new applications of tulip tree (Liriodendron tulipifera L.): Leaf essential oil as apoptotic agent for human glioblastoma. Environ. Sci. Pollut. Res. Int. 2019, 26, 30485–30497. [Google Scholar] [CrossRef] [PubMed]
- Sipos, S.; Moacă, E.A.; Pavel, I.Z.; Avram, Ş.; Crețu, O.M.; Coricovac, D.; Racoviceanu, R.M.; Ghiulai, R.; Pană, R.D.; Şoica, C.M.; et al. Melissa officinalis L. Aqueous Extract Exerts Antioxidant and Antiangiogenic Effects and Improves Physiological Skin Parameters. Molecules 2021, 26, 2369. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Emami, S.A.; Vatani, M.; Tayarani-Najaran, Z. Evaluation of Antioxidant and Anti-Melanogenic Activity of Different Extracts of Aerial Parts of N. Sintenisii in Murine Melanoma B16F10 Cells. Iran. J. Pharm. Res. 2018, 17, 225–235. [Google Scholar]
- Wikiera, A.; Grabacka, M.; Byczyński, Ł.; Stodolak, B.; Mika, M. Enzymatically Extracted Apple Pectin Possesses Antioxidant and Antitumor Activity. Molecules 2021, 26, 1434. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, J.; Park, S.H.; Kim, Y.A.; Park, B.J.; Oh, J.; Sung, G.H.; Aravinthan, A.; Kim, J.H.; Kang, H.; et al. Antiphotoaging and Antimelanogenic Effects of Penthorum chinense Pursh Ethanol Extract due to Antioxidant- and Autophagy-Inducing Properties. Oxid. Med. Cell Longev. 2019, 2019, 9679731. [Google Scholar] [CrossRef]
- Nanni, V.; Canuti, L.; Gismondi, A.; Canini, A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Ciorîță, A.; Zăgrean-Tuza, C.; Moț, A.C.; Carpa, R.; Pârvu, M. The Phytochemical Analysis of Vinca L. Species Leaf Extracts Is Correlated with the Antioxidant, Antibacterial, and Antitumor Effects. Molecules 2021, 26, 3040. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, J.; An, X.H.; Hua, Y.R.; Lai, Y.F.; Zhang, R.; Ahmad, O.; Zhang, Y.; Shang, J. Natural product-based design, synthesis and biological evaluation of 2′,3,4,4′-tetrahydrochalcone analogues as antivitiligo agents. Bioorg Chem. 2019, 87, 523–533. [Google Scholar] [CrossRef]
- Feng, S.; Zhou, Y.; Huang, H.; Lin, Y.; Zeng, Y.; Han, S.; Huang, K.; Liu, Q.; Zhu, W.; Yuan, Z.; et al. Nobiletin Induces Ferroptosis in Human Skin Melanoma Cells Through the GSK3β-Mediated Keap1/Nrf2/HO-1 Signalling Pathway. Front. Genet. 2022, 13, 865073. [Google Scholar] [CrossRef]
- Mokhtari, K.; Pérez-Jiménez, A.; García-Salguero, L.; Lupiáñez, J.A.; Rufino-Palomares, E.E. Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide. Molecules 2020, 25, 4020. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L.; Calpena, A.C.; Garduño-Ramírez, M.L.; Ortiz, R.; Melguizo, C.; Prados, J.C.; Clares, B. Nanoemulsion Strategy for Ursolic and Oleanic Acids Isolates from Plumeria Obtusa Improves Antioxidant and Cytotoxic Activity in Melanoma Cells. Anticancer Agents Med. Chem. 2018, 18, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed cell death detection methods: A systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef]
- Bagati, A.; Moparthy, S.; Fink, E.E.; Bianchi-Smiraglia, A.; Yun, D.H.; Kolesnikova, M.; Udartseva, O.O.; Wolff, D.W.; Roll, M.V.; Lipchick, B.C.; et al. KLF9-dependent ROS regulate melanoma progression in stage-specific manner. Oncogene 2019, 38, 3585–3597. [Google Scholar] [CrossRef]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Khayyati Kohnehshahri, M.; Sarkesh, A.; Mohamed Khosroshahi, L.; HajiEsmailPoor, Z.; Aghebati-Maleki, A.; Yousefi, M.; Aghebati-Maleki, L. Current status of skin cancers with a focus on immunology and immunotherapy. Cancer Cell Int. 2023, 23, 174. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Natarelli, N.; Aleman, S.J.; Mark, I.M.; Tran, J.T.; Kwak, S.; Botto, E.; Aflatooni, S.; Diaz, M.J.; Lipner, S.R. A Review of Current and Pipeline Drugs for Treatment of Melanoma. Pharmaceuticals 2024, 17, 214. [Google Scholar] [CrossRef] [PubMed]
- Scatozza, F.; Moschella, F.; D’Arcangelo, D.; Rossi, S.; Tabolacci, C.; Giampietri, C.; Proietti, E.; Facchiano, F.; Facchiano, A. Nicotinamide inhibits melanoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 2020, 39, 211. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Q.; Zhang, X.; Jin, Q.; Yue, Q.; Li, N.; Liu, H.; Fujimoto, M.; Jin, G. Dihydroartemisinin inhibits melanoma migration and metastasis by affecting angiogenesis. Phytother. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; DeLouise, L.A. Enriching and characterizing cancer stem cell sub-populations in the WM115 melanoma cell line. Biomaterials 2011, 32, 9316–9327. [Google Scholar] [CrossRef]

| Classification | Compound | Source | Cell Line/Animal Model | Dose/Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Flavonoid | Taxifolin | Larix olgensis roots | C57/BL6 mice | 30, 60 mg/kg; 11 d | Inhibition of metastasis and proliferation | ↓PCNA | [38] |
| Plant Extract | Eleutherine bulbosa bulbs | Eleutherine bulbosa | C57BL6 mice | 100, 500, 1000 μg/mL; 14 d | Anti-metastatic property | [39] | |
| Plant Extract | Pine bark | Pinus maritima | A375 | 1, 5, 25, 50 μg/mL; 45 h | Inhibition of metastasis | ↓MMP-9 | [37] |
| Terpenoid | Cynaropicrin | Centaurea drabifolia subsp. detonsa | A375 | 3, 10 μM; 0, 24, 48 h | Inhibition of metastasis | ↑Nrf2 ↓pERK, p65 | [30] |
| Terpenoid | Hinokitiol | Chamaecyparis taiwanensis | B16F10 | 1250 nM; 0, 6, 12 h | Inhibition of metastasis | ↓pAKT, pERK | [40] |
| C57BL/6 mice, BALB/c mice | 1250 nM; 15 d |
| Classification | Compound | Source | Cell Line/Animal Model | Dose/Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| AlgaeProtista | Brown macroalgae | Sargassum cristaefolium (SCE) | B16F10 | 1, 10, 50, 100 μg/mL; 1 h | Initiation of antioxidant properties | ↓ROS | [41] |
| AlgaeProtista | Indonesian Brown algae | Sargassum polycystum, Sargassum cristaefolium, Sargassum aquifolium Turbinaria ornata | B16F10 | 50, 100, 500, 1000, 4000 μg/mL; 16 h | Induction of antioxidant activity | ↓ROS | [42] |
| Animal | Porcupine bezoar | Hystrix Brachyura Bezoar | A375 | 1, 2, 3, 4 μg/mL; 72 h | Induction of antioxidant capacity | ↓ROS | [18] |
| Bacteria | Lactobacillus gasseri BNR17 | B16-F10, HaCaT | 0.16, 0.312, 0.625, 1.25, 2.5, 5, 10% v/v | Induction of antioxidant activity | ↑HO-1, CAT, GPX1, SOD1 ↓ROS | [43] | |
| Benzaldehyde | Cuminaldehyde | Chrysanthemum boreale MAKINO | B16BL6 | 1, 10, 30 μg/mL; 48 h | Induction of antioxidant response and antimelanogenesis properties | ↑Akt, CREB1, MEK1, WNK1, TAT1, STAT3, p38 MAPK, Erk1/2 ↓TYR | [44] |
| Flavonoid | Epicatechin (EC) | Crataegus azarolus L. | B16F10 | 10, 20, 30, 40, 50 μmol/l; 1 h | Antioxidant property | ↓ROS | [45] |
| Flavonoid | Glyasperin A | Macaranga pruinosa | B16 | 2.4, 4.8, 6.0, 7.2 μM | Induction of antioxidant activity | [46] | |
| Fungi | Pleurotus ferulae | Pleurotus ferulae | B16, Eca-109, BGC823, HeLa | 0.5, 1, 2, 5, 10 mg/mL | Induction of antioxidant, antitumor activities | ↓ROS | [47] |
| Phenylethanoid | Acteoside | Lippia citriodora | B16F1, B16F10 | 0.2, 0.4, 0.6, 0.8, 1 mM; 24 h | Induction of antioxidant activity | ↑Akt, CREB1, MEK1, WNK1 ↓ ROS, STAT1, STAT3 | [48] |
| Plant Extract | 4-methoxycinnamic acid, 4-methoxybenzoic acid, methyl linoleate, asterriquinone C-1 | Hygrophila erecta (Brum. F.) Hochr. | B16F10 | 1, 2, 4, 8, 16, 31, 62, 125, 250, 500, 1000 μg/mL; 24 h | Induction of antioxidative role | ↑SOD, Nrf2 ↓ROS, NO | [49] |
| Plant Extract | Aged garlic extract (AGE) | Allium sativum L. | M14 WT | 1, 5, 10 mg/mL; 40 min | Induction of antioxidant activity | ↓ROS | [21] |
| Plant Extract | Cinnamomum loureirii Nees | Cinnamomum loureirii Nees | G361 | 1 mg/mL; 30 min | Induction of antioxidant activity | ↓ROS | [28] |
| Plant Extract | Curcuma longa, Lycium barbarum, Equisetum arvense, Vitis vinifera, Rosmarinus officinalis | Curcuma longa, Lycium barbarum, Equisetum arvense, Vitis vinifera, Rosmarinus officinalis | WM-266-4 | 0.001, 0.01, 0.1, 1, 5, 10, 20, 100 μg/mL; 24 h | Induction of antioxidant activity | ↓ROS | [50] |
| Plant Extract | Ginger, hemp | Zingiber officinale, Cannabis sativa L. | B16F10 | 0.01, 0.1, 1, 5, 10, 20, 50 mg/mL; 24 h | Induction of antioxidant activity | ↓ROS | [51] |
| Plant Extract | Garcinia atroviridis | Garcinia atroviridis Griff. ex. T. Anderson | B16F10 | 62.5, 125, 250, 500, 1000 μg/mL | Induction of pro-antioxidant activity | ↓ROS | [52] |
| Plant Extract | Hymenaea courbaril L. | Hymenaea courbaril L. | B16F10 | 250 μg/mL | Induction of antioxidant activity | ↓ROS | [53] |
| Plant Extract | Laminaria ochroleuca, Porphyra umbilicalis, Gelidium corneum | Laminaria ochroleuca, Porphyra umbilicalis, Gelidium corneum | G-361 | 25, 50, 75, 100, 150, 200, 300, 400, and 500 μg/mL; 16 h | Induction of antioxidant property | ↓ROS | [54] |
| Plant Extract | Lawsonia inermis L. | Lawsonia inermis L. | C57BL/6 mice | 500, 1000 mg/kg; 40 d | Exhibition of optimum antioxidant activity and protection against oxidative stress | ↓GSH-Px, SOD, CAT | [55] |
| Plant Extract | Leonotis nepetifolia (L.) R. Br. root | Leonotis nepetifolia (L.) R. Br. | A375 | 10 μg/mL; 16 h | Possession of antioxidant activity | ↓ROS, Mdm-2 | [29] |
| Plant Extract | Liriodendron tulipifera L. | Liriodendron tulipifera L. | A375 | 5 mg/mL; 72 h | Induction of antioxidant activity | ↓ROS | [56] |
| Plant Extract | Melissa officinalis L. | Melissa officinalis L. | HaCaT, A375 | 0.1, 0.5, 1, 3, 5 mg/mL; 20 min | Induction of antioxidant activity | ↓ROS | [57] |
| Plant Extract | Nepeta sintenisii Born. | Nepeta sintenisii Born. | B16F10 | 50 μg/mL; 24 h | Induction of antioxidant activity | ↓ROS, TYR | [58] |
| Plant Extract | Parsley | Parsley | A375 | 10, 30, 60 μg/mL; 72 h | Induction of antioxidant and anti-inflammatory activity | ↑c-caspase-3 ↓ IL-10 | [24] |
| Plant Extract | Pectin | Dried apple pomace | HT-29, B16F10 | 0.2, 0.4, 0.6, 0.8, 1 mg/mL; 24 h | Induction of antioxidant activity | ↓ROS | [59] |
| Plant Extract | Penthorum chinense Pursh | Penthorum chinense Pursh | B16-F10, HaCaT | 50, 100 μg/mL; 24 h | Induction of antioxidative effect | ↓ROS, IL-6, COX-2, p-ERK, p-p38, p-JNK, MMP | [60] |
| Plant Extract | Spartium junceum L. | Spartium junceum L. | B16F10 | 4, 8, 10 mg/mL; 4, 24, 48 h | Induction of antioxidant activity | ↓ MIFT | [61] |
| Plant Extract | Stryphnodendron adstringens (Mart.) Coville (Fabaceae) | Stryphnodendron adstringens (Mart.) Coville (Fabaceae) | B16F10Nex-2 | 10, 50 μg/mL; | Induction of antioxidant activity | [35] | |
| Plant Extract | Thai water lily | Nymphaea stellate | B16F10 | 200, 400, 600, 800, 1000 μg/mL; 24 h | Induction of antioxidant activities | ↓ROS | [36] |
| Plant Extract | Vinca minor, V. herbacea, V. major, V. major var. variegata | Vinca minor, V. herbacea, V. major, V. major var. variegata | HaCaT, A375 | 0.09, 0.5, 1, 2, 3%; 24, 72 h | Induction of antioxidant, antibacterial and antitumor activity | [62] | |
| Polyphenol | 2′,3,4,4′-tetrahydrochalcone | Vernohia anthelmintica (L.) willd | B16F10 | 1, 10, 20, 40 μM/L; 30 min | Induction of antioxidant activity | ↓ROS | [63] |
| C57BL/6 mice | 8.5, 10 μg; 40 d | ||||||
| Polyphenol | Nobiletin | Citrus unshiu Markovich | SK-MEL-28 | 5, 15, 45 μM; 30 min | Induction of antioxidant activity | ↑Keap1 ↓Nrf2, ROS | [64] |
| Terpenoid | Cynaropicrin | Centaurea drabifolia subsp. detonsa | A375 | 3, 10 μM; 0, 24, 48 h | Inhibition of metastasis | ↑Nrf2 ↓pERK, p65 | [30] |
| Triterpenoid | Maslinic acid | Olea europaea L. | A10, B16F10 | 10.6, 21.6, 42.3, 84.6 μM; 24 h | Induction of antioxidant activity | ↓ROS, CAT, G6PDH, SOD, GST, GPX, GR | [65] |
| Triterpenoid | Oleanolic acid, Ursolic acid | Plumeria obtusa | B16 | 2 mg/mL; 90 min | Induction of antioxidant activity | ↓ROS | [66] |
| Classification | Compound | Source | Cell Line/Animal Model | Dose/Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Plant Extract | Melissa officinalis L. | Melissa officinalis L. | HaCaT, A375 | 20, 100, 250, 500, 1000 μg/mL; 24 h | Induction of antiangiogenic and antioxidant activity | [57] | |
| SKH-1 mice | 5 mg/mL; 14 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.; An, J.; Lee, D.; Kang, H.N.; Kang, S.; Ahn, C.-H.; Syahputra, R.A.; Ribeiro, R.I.M.A.; Kim, B. Natural Products for Melanoma Therapy: From Traditional Medicine to Modern Drug Discovery. Plants 2025, 14, 951. https://doi.org/10.3390/plants14060951
An S, An J, Lee D, Kang HN, Kang S, Ahn C-H, Syahputra RA, Ribeiro RIMA, Kim B. Natural Products for Melanoma Therapy: From Traditional Medicine to Modern Drug Discovery. Plants. 2025; 14(6):951. https://doi.org/10.3390/plants14060951
Chicago/Turabian StyleAn, Soojin, Jeongeun An, Dain Lee, Han Na Kang, Sojin Kang, Chi-Hoon Ahn, Rony Abdi Syahputra, Rosy Iara Maciel A. Ribeiro, and Bonglee Kim. 2025. "Natural Products for Melanoma Therapy: From Traditional Medicine to Modern Drug Discovery" Plants 14, no. 6: 951. https://doi.org/10.3390/plants14060951
APA StyleAn, S., An, J., Lee, D., Kang, H. N., Kang, S., Ahn, C.-H., Syahputra, R. A., Ribeiro, R. I. M. A., & Kim, B. (2025). Natural Products for Melanoma Therapy: From Traditional Medicine to Modern Drug Discovery. Plants, 14(6), 951. https://doi.org/10.3390/plants14060951








