Effects of Varying Nitrogen Concentrations on the Locule Number in Tomato Fruit
Abstract
1. Introduction
2. Results
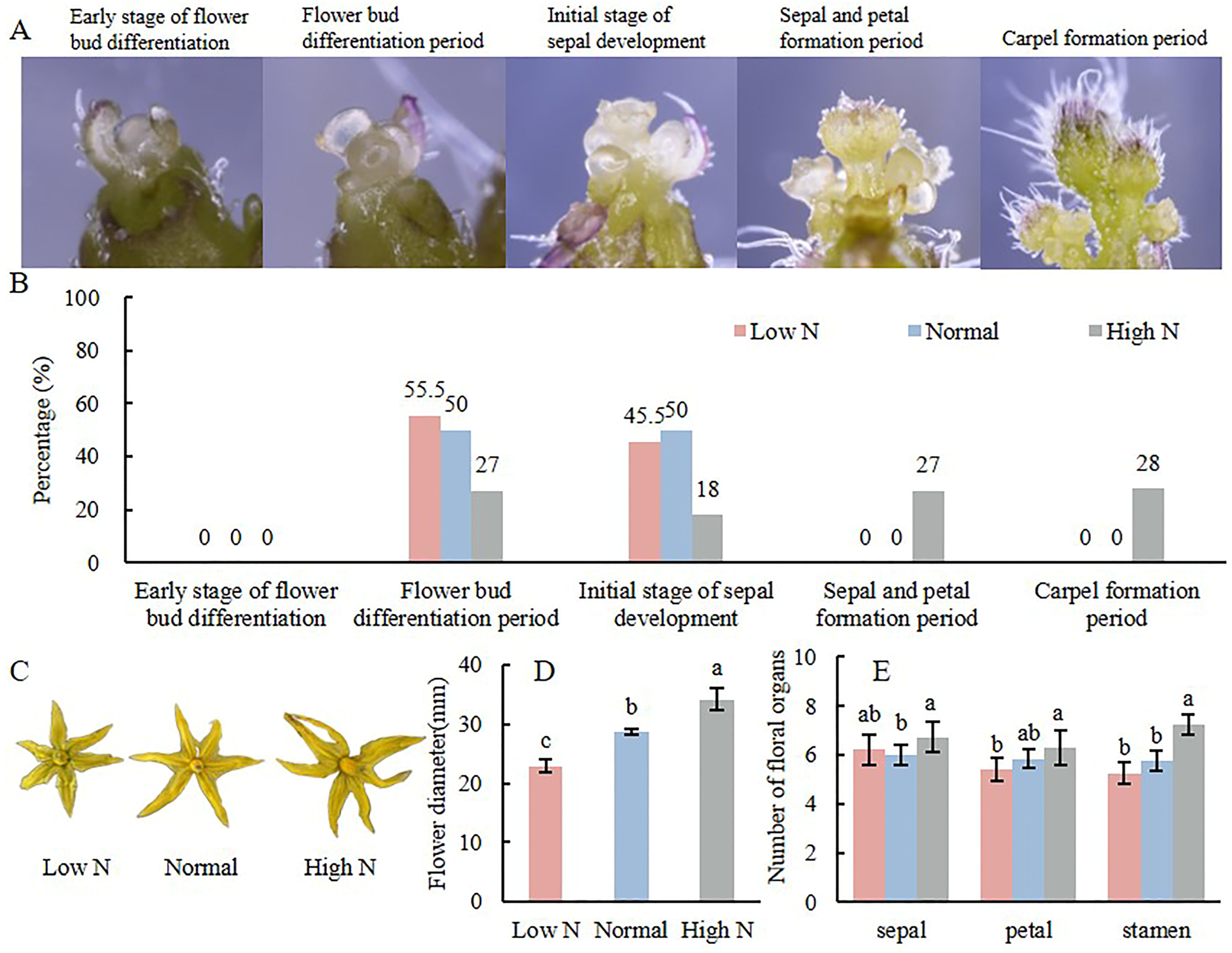
2.1. Effects of Varying Nitrogen Concentration on Tomato Flower Development
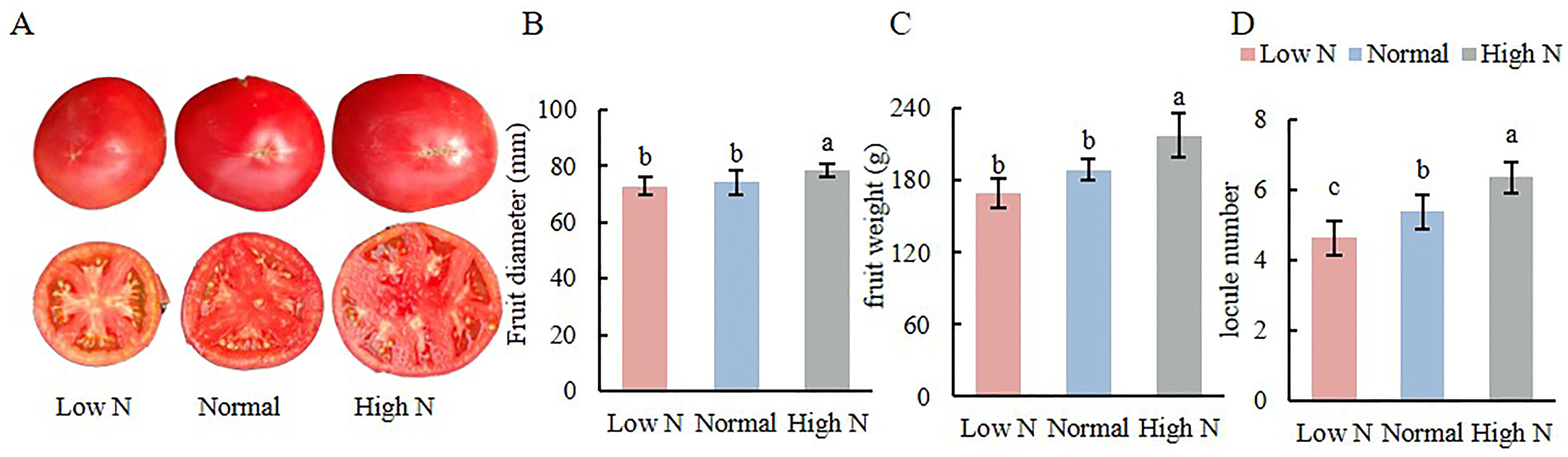
2.2. Effects of Varying Nitrogen Concentration on Tomato Fruit
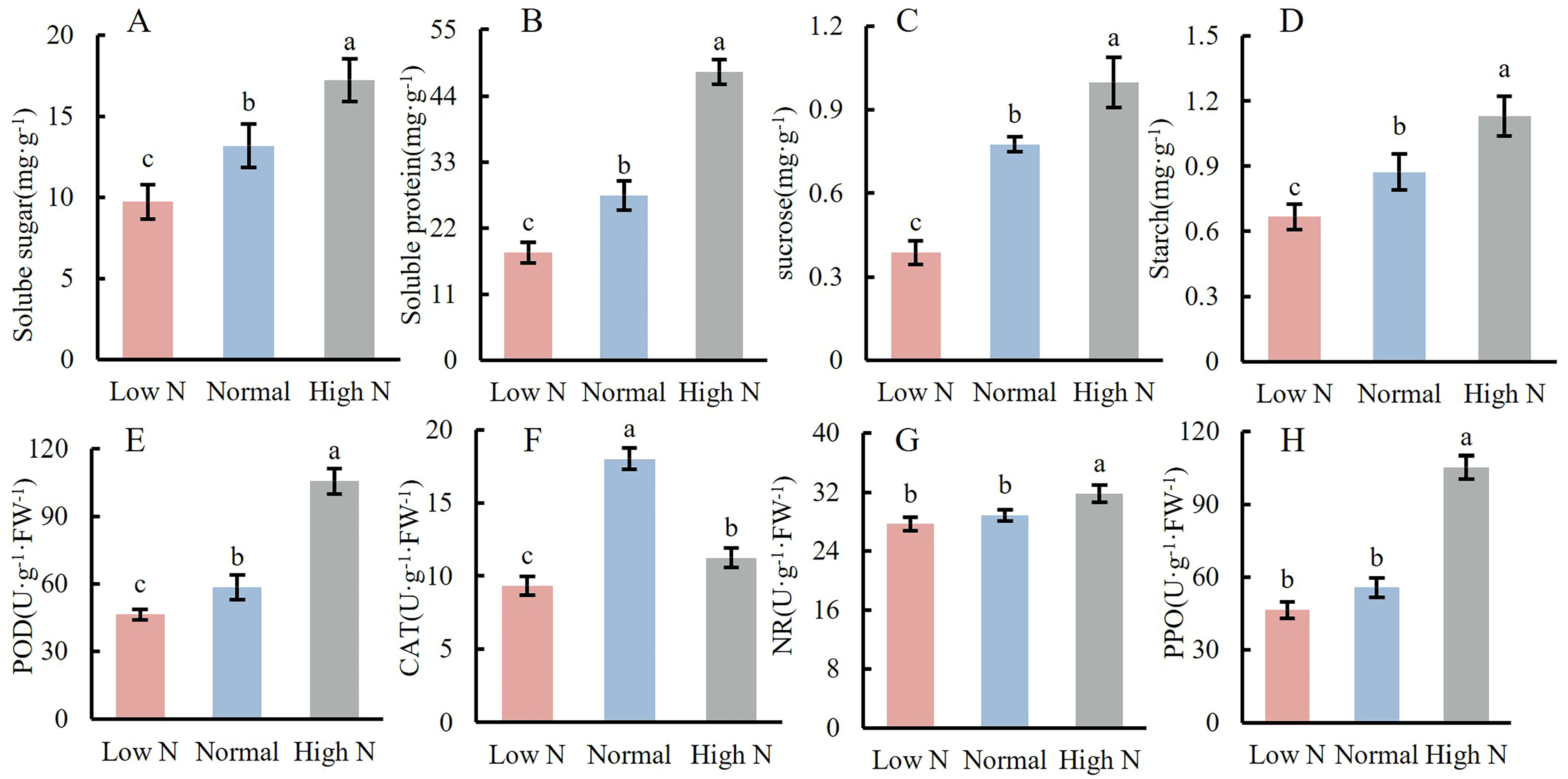
2.3. Effects of Varying Nitrogen Concentration on Nutrient Content and Enzyme Activity in the Stem Apex of Tomato Seedlings
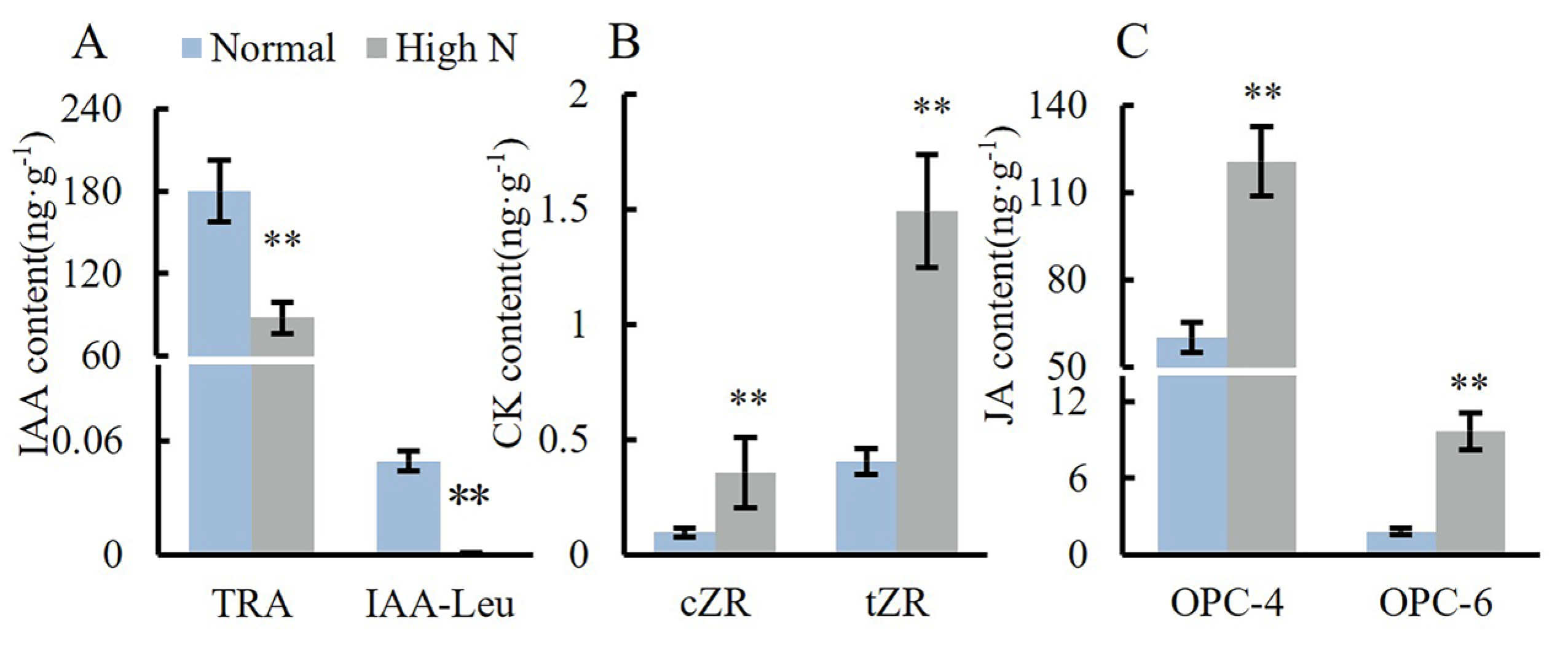
2.4. Effects of Varying Nitrogen Concentration on Hormone Content in the Stem Apex of Tomato Seedlings
2.5. Transcriptome Data Analysis
2.6. Effects of Varying Nitrogen Concentration on Expression of Related Genes in the Stem Apex of Tomato Seedlings
2.7. Analysis of Expression Patterns of Candidate Genes in Different Tissues
3. Discussion
3.1. Effects of Varying Nitrogen Concentration on Tomato Flower Development and Fruit
3.2. Effects of Varying Nitrogen Concentration on Nutrient Content, Enzyme Activity and Hormone Content in the Stem Apex of Tomato Seedlings
3.3. Effects of Varying Nitrogen Concentration on Gene Expression in the Stem Apex of Tomato Seedlings
4. Materials and Methods
4.1. Plant Materials
4.2. Flower and Fruit Phenotypic Analysis
4.3. Physiological Index Analysis
4.4. Transcriptome Sequencing and Bioinformatics Analysis
4.5. RNA Extraction and Related Gene Expression Analysis
4.6. Analysis of Gene Expression Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cong, B.; Barrero, L.S.; Tanksley, S.D. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 2008, 40, 800–804. [Google Scholar] [CrossRef]
- Xu, C.; Liberatore, K.L.; MacAlister, C.A.; Huang, Z.; Chu, Y.; Jiang, K.; Brooks, C.; Ogawa-Ohnishi, M.; Xiong, G.; Pauly, M.; et al. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 2015, 47, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering quantitative trait variation for crop improvement by genome editing. Cell 2017, 171, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.; Ranc, N.; Botton, E.; Berard, A.; Rolland, S.; Duffé, P.; Carretero, Y.; Le Paslier, M.C.; Delalande, C.; Bouzayen, M.; et al. Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL. Plant Physiol. 2011, 156, 2244–2254. [Google Scholar] [CrossRef]
- Su, D.; Wen, L.; Xiang, W.; Shi, Y.; Lu, W.; Liu, Y.; Xian, Z.; Li, Z. Tomato transcriptional repressor SlBES1.8 influences shoot apical meristem development by inhibiting the DNA binding ability of SlWUS. Plant J. 2022, 110, 482–498. [Google Scholar] [CrossRef]
- Song, S.; Huang, B.; Pan, Z.; Wang, Y.; Li, X.; Zhang, X.; Liu, Y.; Wang, J.; Li, J.; Li, H. The SlTPL3–SlWUS module regulates multi-locule formation in tomato by modulating auxin and gibberellin levels in the shoot apical meristem. J. Integr. Plant Biol. 2022, 64, 2150–2167. [Google Scholar] [CrossRef]
- Bollier, N.; Sicard, A.; Leblond-Castaing, J.; Schoof, H.; Lenhard, M.; Chevalier, C.; Laufs, P. At-MINI ZINC FINGER2 and Sl-INHIBITOR OF MERISTEM ACTIVITY, a conserved missing link in the regulation of floral meristem termination in Arabidopsis and tomato. Plant Cell 2018, 30, 83–100. [Google Scholar] [CrossRef]
- Yuste-Lisbona, F.J.; Fernandez-Lozano, A.; Pineda, B.; Bretones, S.; Ortiz-Atienza, A.; Garcia-Sogo, B.; Muller, N.A.; Angosto, T.; Capel, J.; Moreno, V.; et al. ENO regulates tomato fruit size through the floral meristem development network. Proc. Natl. Acad. Sci. USA 2020, 117, 8187–8195. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Xiang, H.; Li, T. Transcriptome analysis reveals regulatory effects of exogenous gibberellin on locule number in tomato. Plant Growth Regul. 2020, 91, 407–417. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.L. Regulation effects of exogenous gibberellin acid (GA3) on the formation of tomato (Solanum lycopersicum) ovary locule and fasciated transcription. Afr. J. Biotechnol. 2017, 16, 13732–13738. [Google Scholar]
- Liu, B.; Liu, X.; Yang, S.; Li, C.; Wang, X.; Zhang, Z.; Zhang, X. Silencing of the gibberellin receptor homolog, CsGID1a, affects locule formation in cucumber (Cucumis sativus) fruit. New Phytol. 2015, 210, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Wu, J.; Han, X.; Li, Y.; Shi, X.; Li, J.; Li, C.; Liu, Y.; Li, Z. Auxin homeostasis is maintained by sly-miR167-SlARF8A/B-SlGH3.4 feedback module in the development of locular and placental tissues of tomato fruits. New Phytol. 2024, 241, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Santos, J.S.; Souza, J.T.; Almeida, O.C.; Pungartnik, C. Moniliophthora perniciosa, the causal agent of witches’ broom disease of cacao, interferes with cytokinin metabolism during infection of Micro-Tom tomato and promotes symptom development. New Phytol. 2021, 231, 365–381. [Google Scholar]
- Li, Y.; Li, T.L.; Wang, D. Correlation between endogenous hormones of stem apices and fruit locule numbers in tomatoes during floral bud differentiation stages. Agric. Sci. China 2008, 7, 447–454. [Google Scholar] [CrossRef]
- Wu, J.; Sun, W.; Sun, C.; Xu, C.; Li, S.; Li, P.; Xu, H.; Zhu, D.; Li, M.; Yang, L.; et al. Cold stress induces malformed tomato fruits by breaking the feedback loops of stem cell regulation in floral meristem. New Phytol. 2023, 237, 2268–2283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, M.; Xiang, H.; Li, T. Low overnight temperature-induced gibberellin accumulation increases locule number in tomato. Int. J. Mol. Sci. 2019, 20, 3042. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, X.; Bing, L.; Song, J.; Qi, Y.; Han, Q.; Yang, Y.; Lin, D. Responses of Growth, Enzyme Activity, and Flower Bud Differentiation of Pepper Seedlings to Nitrogen Concentration at Different Growth Stages. Agronomy 2024, 14, 2270. [Google Scholar] [CrossRef]
- Bibiano Ferreira, R.B.; Leonel, S.; Pacce Pereira Lima, G.; Leonel, M.; Otávio Minatel, I.; Mirellys Azevedo Souza, J.; Charles Monteiro, G.; Souza Silva, M. Contents of nitrogen compounds during bud break and peach tree performance in response to bud burst-inducing products. Sci. Hortic. 2022, 305, 111388. [Google Scholar] [CrossRef]
- Wan, C.; Mi, L.; Chen, B.; Li, J.; Huo, H.; Xu, J.; Chen, X. Effects of nitrogen during nursery stage on flower bud differentiation and early harvest after transplanting in strawberry. Braz. J. Bot. 2017, 41, 1–10. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Xu, G. How Does Nitrogen Shape Plant Architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Wu, Y.; Wu, W.; Lyu, L.; Li, W. Effects of Nitrogen Application Level on the Physiological Characteristics, Yield and Fruit Quality of Blackberry. Sci. Hortic. 2023, 313, 111915. [Google Scholar] [CrossRef]
- Andrés, F.; Kinoshita, A.; Kalluri, N.; Fernández, V.; Coupland, G. The sugar transporter SWEET10 acts downstream of flowering locus T during floral transition of Arabidopsis thaliana. BMC Plant Biol. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, X.; Liu, S.; Zhang, X.; Li, Y.; Shang, W.; Song, J.; Tian, J.; Li, X.; Xing, L. Comparative Proteomic and Metabonomic Profiling of Buds with Different Flowering Capabilities Reveal Novel Regulatory Mechanisms of Flowering in Apple. Plants 2023, 12, 3959. [Google Scholar] [CrossRef]
- Shang, C.; Cao, X.; Tian, T.; Hou, Q.; Wen, Z.; Qiao, G.; Wen, X. Cross-Talk between Transcriptome Analysis and Dynamic Changes of Carbohydrates Identifies Stage-Specific Genes during the Flower Bud Differentiation Process of Chinese Cherry (Prunus pseudocerasus L.). Int. J. Mol. Sci. 2022, 23, 15562. [Google Scholar] [CrossRef]
- Xu, Y.X.; Li, J.J.; Wang, P.Y.; Zhang, X.; Liu, Y.; Wang, J. The exploration of flowering mechanisms in cherry plants. Plants 2023, 12, 3980. [Google Scholar] [CrossRef]
- Ullah, F.; Yi, M. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar]
- Zhou, B.Y.; Lu, X.Y.; Li, J.J.; Wang, Y.; Li, M.; Zhu, D. RNA-seq analysis of apical meristem reveals integrative regulatory network of ROS and chilling potentially related to flowering in Litchi chinensis. Sci. Rep. 2017, 7, 10183. [Google Scholar]
- Iqbal, A.; Dong, Q.; Wang, X.R.; Gui, H.P.; Zhang, H.H.; Zhang, X.L.; Song, M.Z. High Nitrogen Enhances Drought Tolerance in Cotton Through Antioxidant Enzymatic Activities, Nitrogen Metabolism and Osmotic Adjustment. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Rong, C.; Liu, Y.; Chang, Z.; Liu, Z.; Ding, Y.; Ding, C. Cytokinin oxidase/dehydrogenase family genes exhibit functional divergence and overlap in rice growth and development, especially in control of tillering. J. Exp. Bot. 2022, 73, 3552–3568. [Google Scholar] [CrossRef]
- Tokunaga, H.; Kojima, M.; Kuroha, T.; Ishida, T.; Sakakibara, H. Arabidopsis lonely guy (log) multiple mutants reveal a central role of the log-dependent pathway in cytokinin activation. Plant J. 2012, 69, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jameson, G.B.; Guo, Y.; Song, J.; Jameson, P.E. The LONELY GUY gene family: From mosses to wheat, the key to the formation of active cytokinins in plants. Plant Biotechnol. J. 2022, 20, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nat. Commun. 2016, 7, 13179. [Google Scholar] [CrossRef]
- Wang, R.; Zhong, Y.; Han, J.; Huang, L.; Wang, Y.; Shi, X.; Li, M.; Zhuang, Y.; Ren, W.; Liu, X. Nin-like protein3.2 inhibits repressor Aux/IAA14 expression and enhances root biomass in maize seedlings under low nitrogen. Plant Cell 2024, 36, 4388–4403. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, J.; Liu, C.X.; Jiang, J.B.; Yang, H.H.; Li, J.F. Genetic characteristics and QTL analysis of the soluble sugar content in ripe tomato fruits. Sci. Hortic. 2021, 276, 109785. [Google Scholar] [CrossRef]
- Alcázar, A.; Jurado, J.M.; Martín, M.J.; Pablos, F.; González, A.G. Enzymatic-spectrophotometric determination of sucrose in coffee beans. Talanta 2005, 67, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.J.; Kasote, D.M.; Jayaprakasha, G.K.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Effect of production system and inhibitory potential of aroma volatiles on polyphenol oxidase and peroxidase activities of tomatoes. J. Agric. Food Chem. 2020, 101, 307–314. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-Espinoza, M.C. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem. 2016, 194, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Poonnachit, U.; Darnell, R. Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Ann. Bot. 2004, 93, 399–405. [Google Scholar] [CrossRef]
- Cai, B.D.; Zhu, J.X.; Gao, Q.; Luo, D.; Yuan, B.F.; Feng, Y.Q. Rapid and high-throughput determination of endogenous cytokinins in Oryza sativa by bare Fe₃O₄ nanoparticles-based magnetic solid-phase extraction. J. Chromatogr. A 2014, 1340, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Q.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef] [PubMed]


| Item | Soluble Protein | Starch | Sucrose | Soluble Sugar | POD | NR | CAT | PPO |
|---|---|---|---|---|---|---|---|---|
| Flower size | 0.94 ** | 0.911 ** | 0.981 ** | 0.898 ** | 0.934 ** | 0.834 ** | 0.211 | 0.879 ** |
| Fruit size | 0.947 ** | 0.937 ** | 0.903 ** | 0.971 ** | 0.897 ** | 0.802 ** | 0.202 | 0.903 ** |
| Fruit weight | 0.942 ** | 0.869 ** | 0.820 ** | 0.916 ** | 0.899 ** | 0.794 ** | 0.071 | 0.919 ** |
| Locule number | 0.850 ** | 0.747 * | 0.708 * | 0.872 ** | 0.856 ** | 0.721 | 0.180 | 0.811 ** |
| Treatment | KNO3 | Ca(NO3)2·4H2O | NaH2PO4·4H2O | NH4NO3 | CaCl2 | MgSO4 | Total Amount of Fertilizer | ||
|---|---|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | |||||||
| Normal | 301 | 912 | 395.5 | -- | -- | 400 | 150 | 180 | 140 |
| High N | 301 | 912 | 395.5 | 286 | -- | 400 | 250 | 180 | 140 |
| Low N | 301 | 70 | 395.5 | -- | 748 | 400 | 50 | 180 | 140 |
| Gene | Primer Sequence |
|---|---|
| ABP19a-F | CCCAAGTGGTGTCATCCCAT |
| ABP19a-R | AGGAATGTTGTTGCCGCAAC |
| IAA2-F | AAGTAGTTGGATGGCCACCG |
| IAA2-R | TACGTCACCAGCAAGCATCC |
| IAA17-F | TGCCTGGTGGAGCAAGAAAA |
| IAA17-R | TAAGGTGCACCATCCATGCT |
| PRX44-like-F | AAGTTCTGCAACGGCGTTTC |
| PRX44-like-R | TCTCCAGCACTCCCCACTAA |
| LOG3-F | GGAGGAGGTAGCATAGGCCT |
| LOG3-R | TACCGGCTTATCGTGGATGC |
| LOG6-F | AAGTGATAACCTGGGCGCAA |
| LOG6-R | CCAAGTTGTTCCGTCTCCCA |
| LOG10-F | ATACACAGGCTTGGTCGTGG |
| LOG10-R | CCTTGCGGAGAAAAACCTGC |
| CKX-F | AATCGATGGTCAGTGGCGTT |
| CKX-R | GCCAGGTGCAAGGACATACT |
| ACTIN-F | 5′-GATCAGCGTATCCTTCAGAG-3′ |
| ACTIN-R | 5′-GGCATTGTAGCAGAGAAAAC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Li, J.; Tian, L.; Sun, H.; Miao, Y.; Bai, L.; Hou, L.; Li, T. Effects of Varying Nitrogen Concentrations on the Locule Number in Tomato Fruit. Plants 2025, 14, 952. https://doi.org/10.3390/plants14060952
Sun M, Li J, Tian L, Sun H, Miao Y, Bai L, Hou L, Li T. Effects of Varying Nitrogen Concentrations on the Locule Number in Tomato Fruit. Plants. 2025; 14(6):952. https://doi.org/10.3390/plants14060952
Chicago/Turabian StyleSun, Meihua, Jing Li, Linlin Tian, Huixian Sun, Yanxiu Miao, Longqiang Bai, Leiping Hou, and Tianlai Li. 2025. "Effects of Varying Nitrogen Concentrations on the Locule Number in Tomato Fruit" Plants 14, no. 6: 952. https://doi.org/10.3390/plants14060952
APA StyleSun, M., Li, J., Tian, L., Sun, H., Miao, Y., Bai, L., Hou, L., & Li, T. (2025). Effects of Varying Nitrogen Concentrations on the Locule Number in Tomato Fruit. Plants, 14(6), 952. https://doi.org/10.3390/plants14060952






