Regulating the Vascular Cambium: Do Not Forget the Vascular Ray Initials and Their Derivatives
Abstract
:1. The Significance of Vascular Land Plants’ Meristems
2. Primary Meristems
3. Secondary Meristems
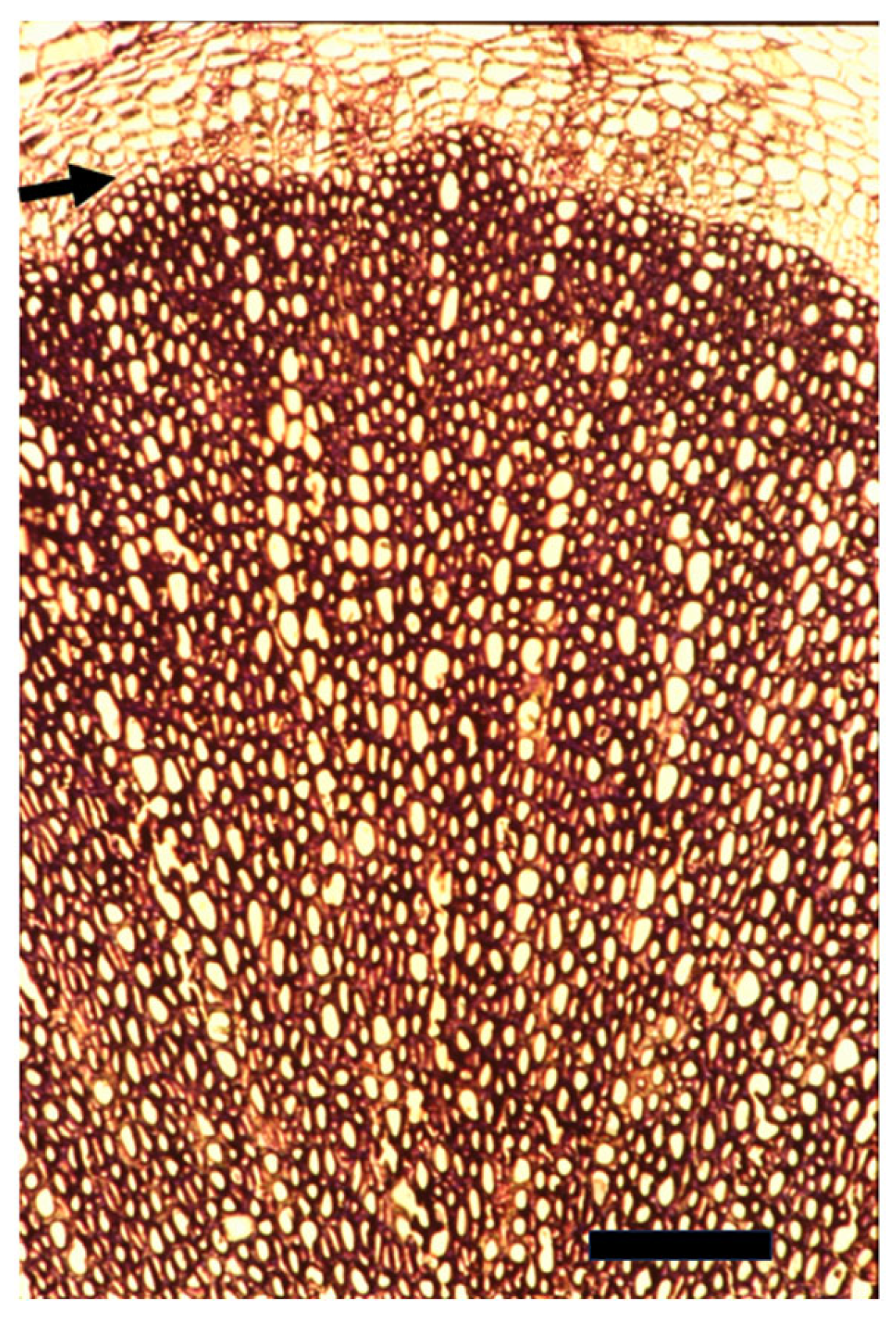
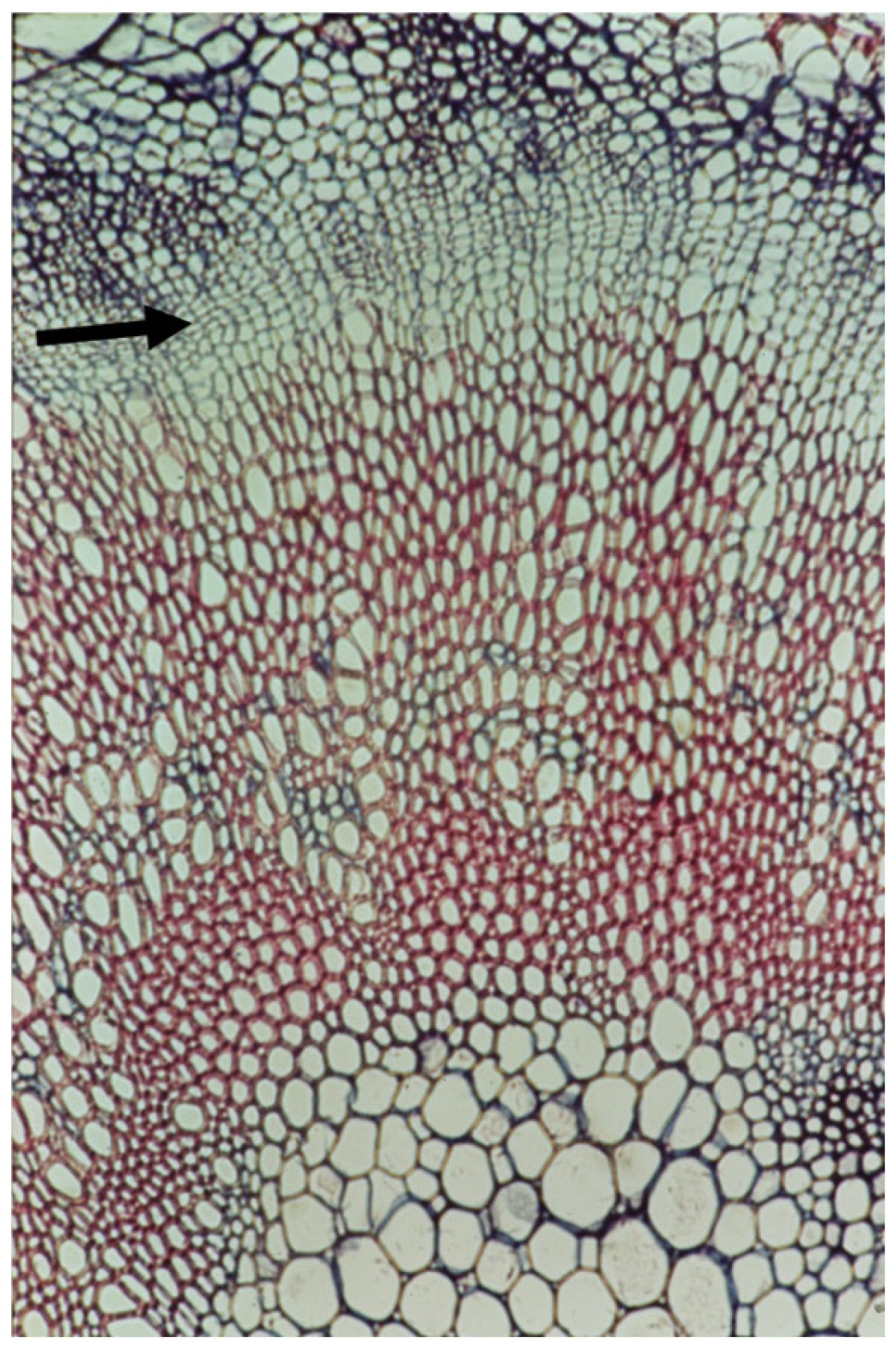
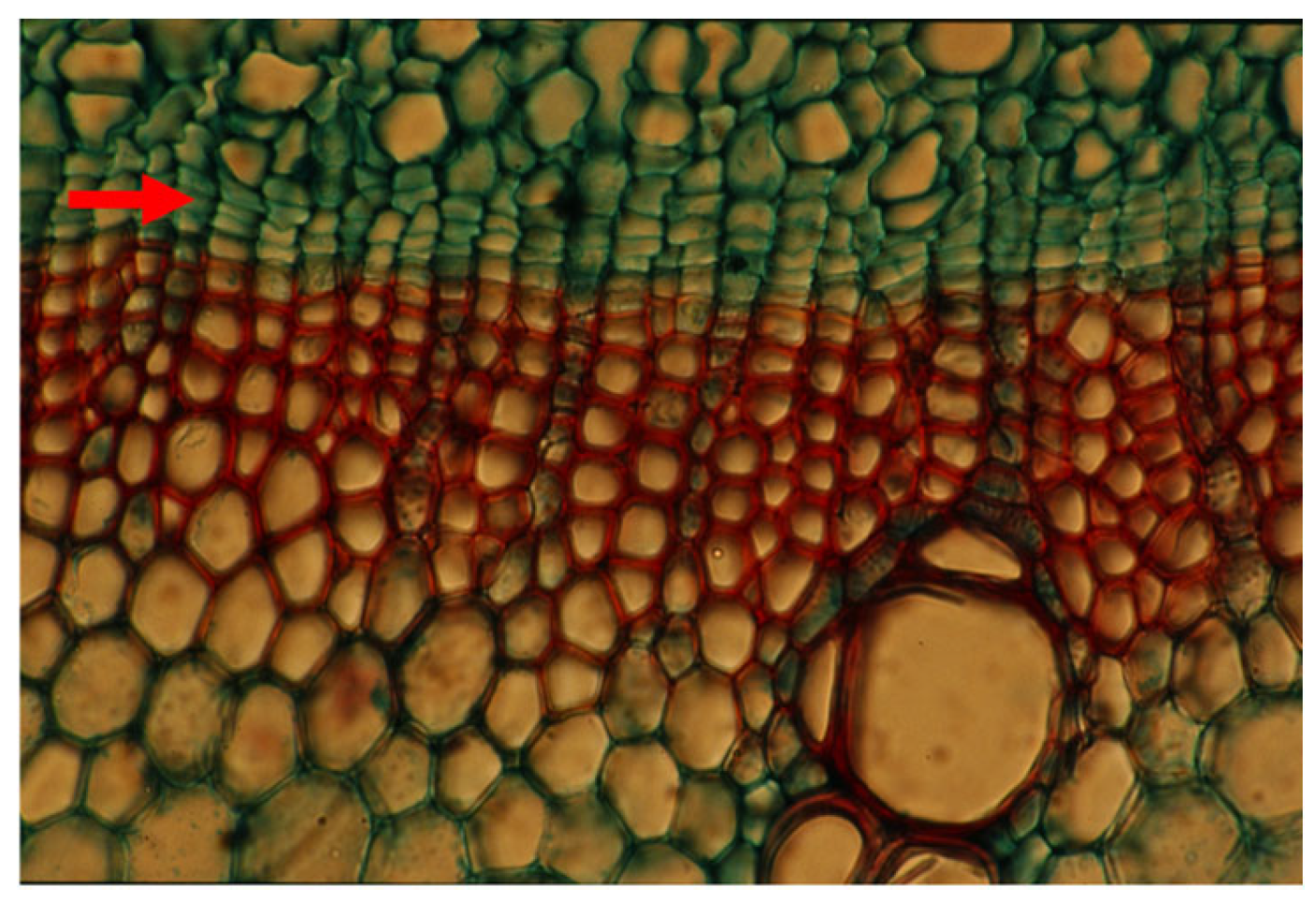
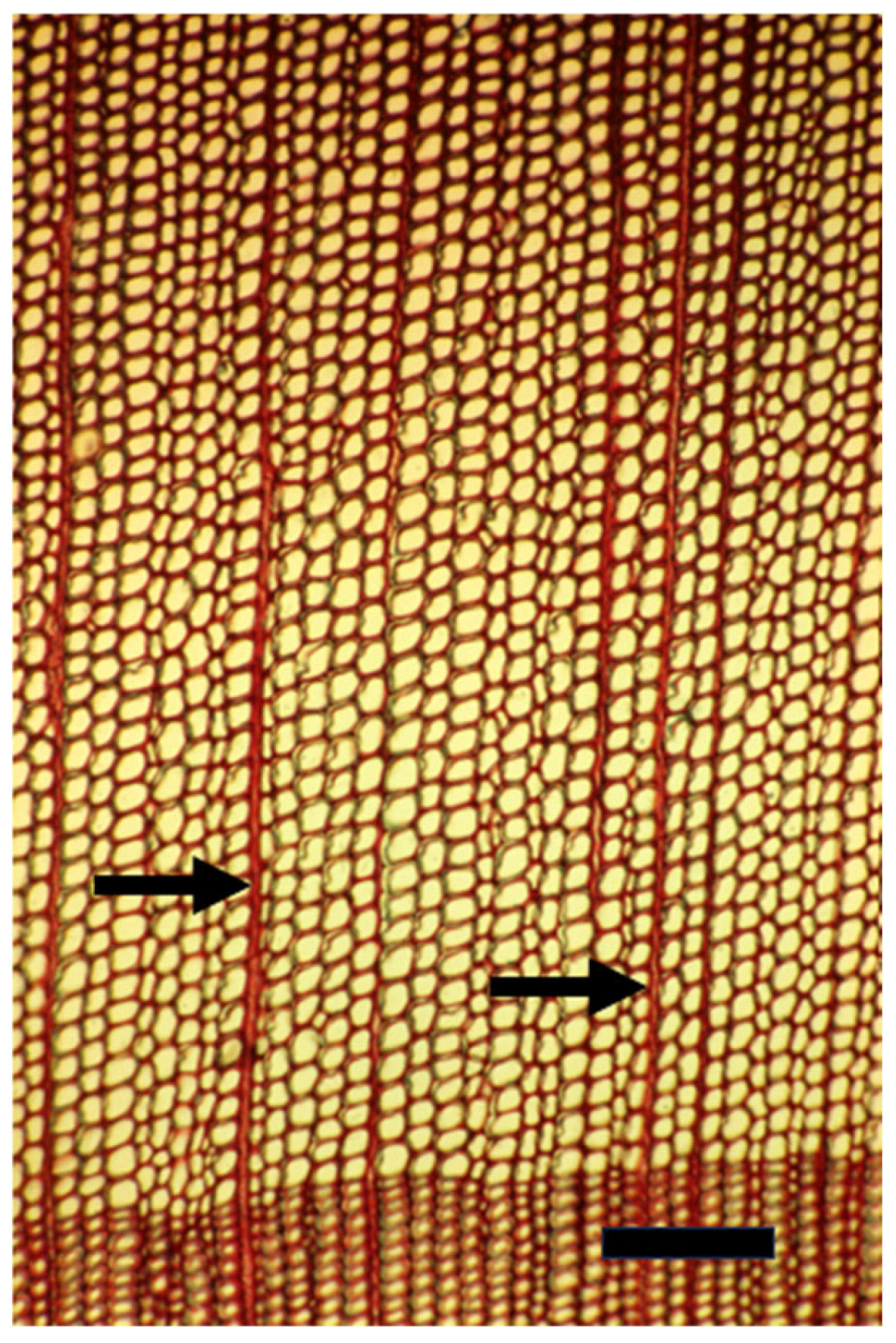
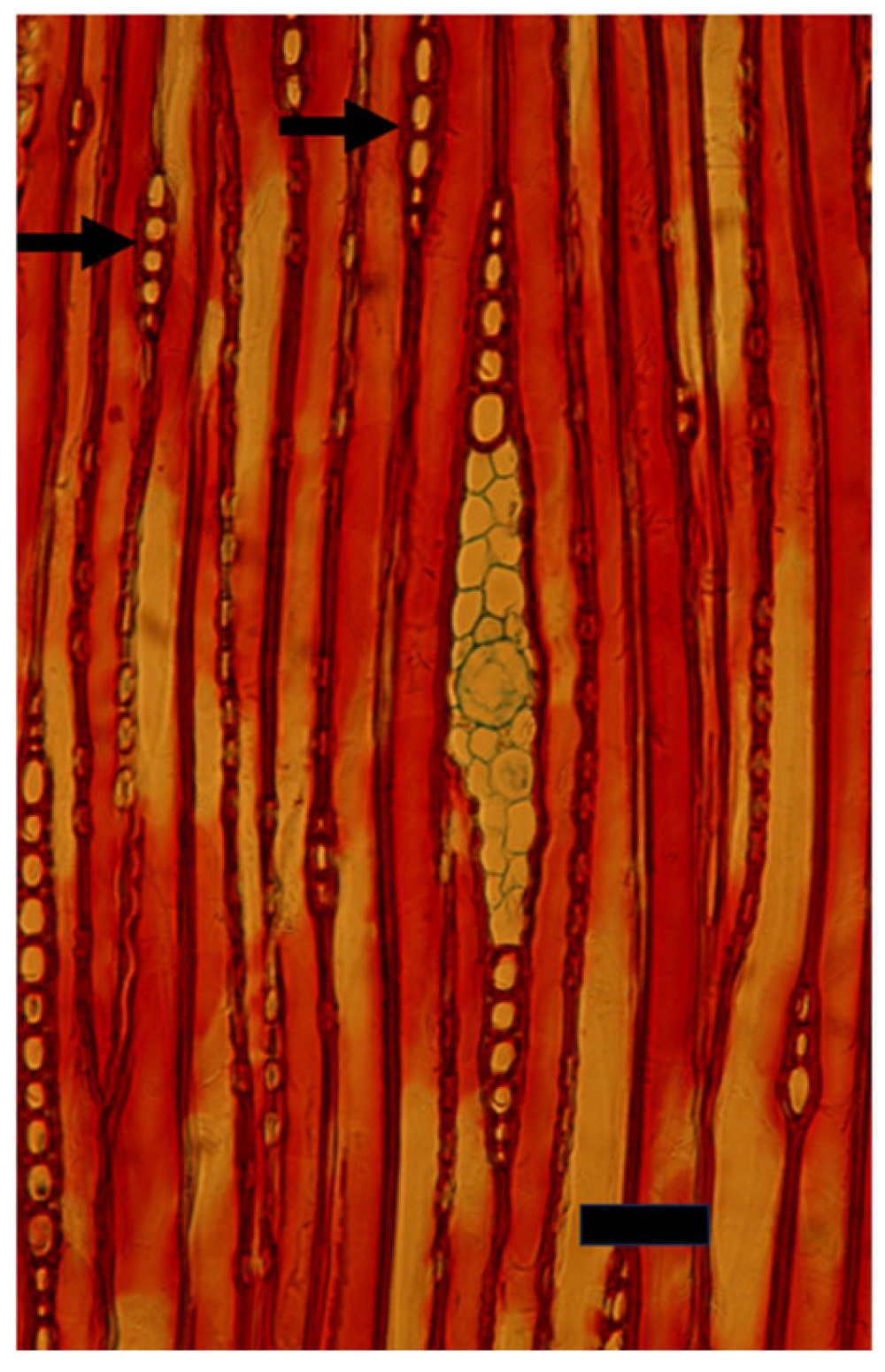
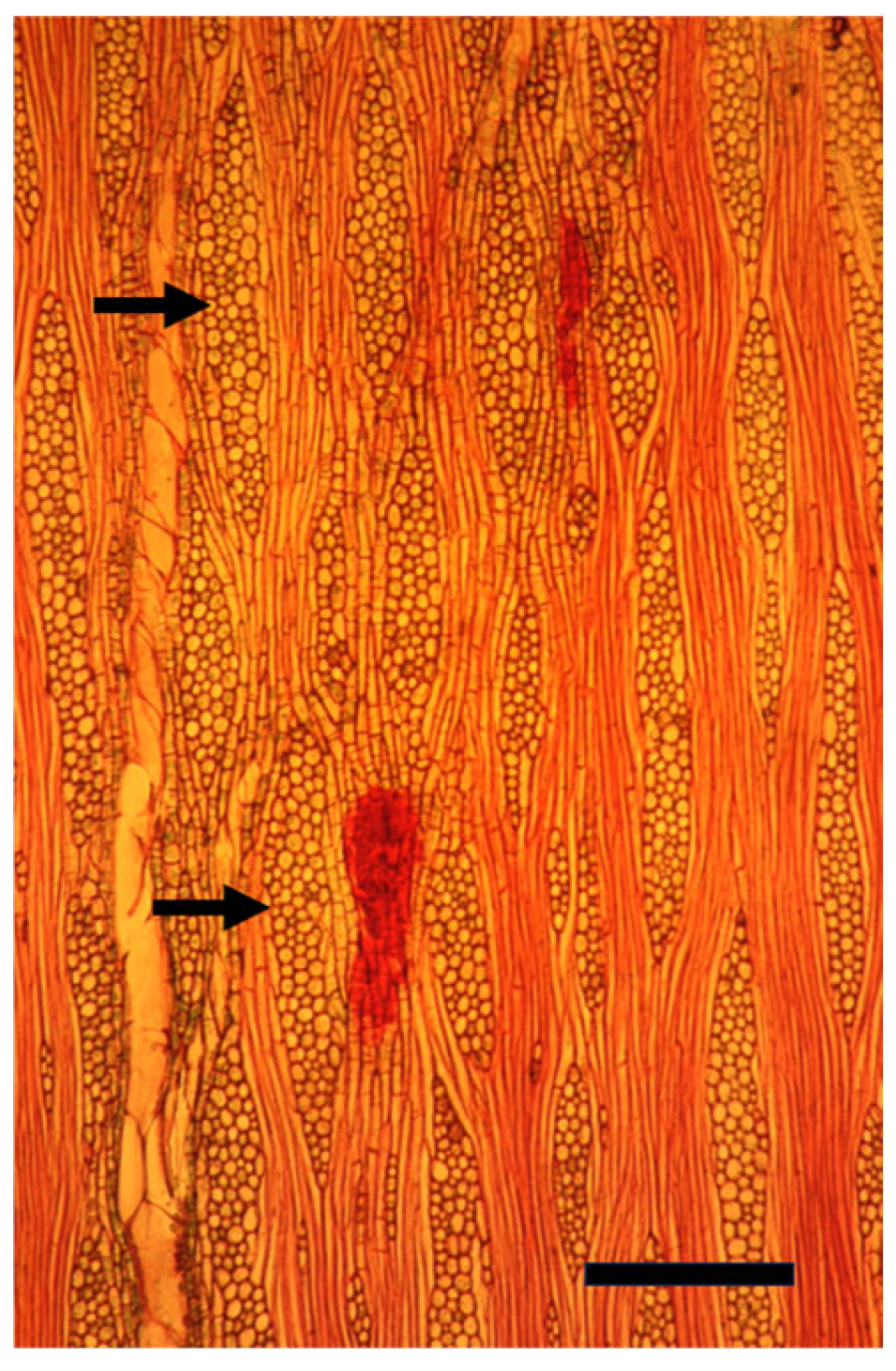
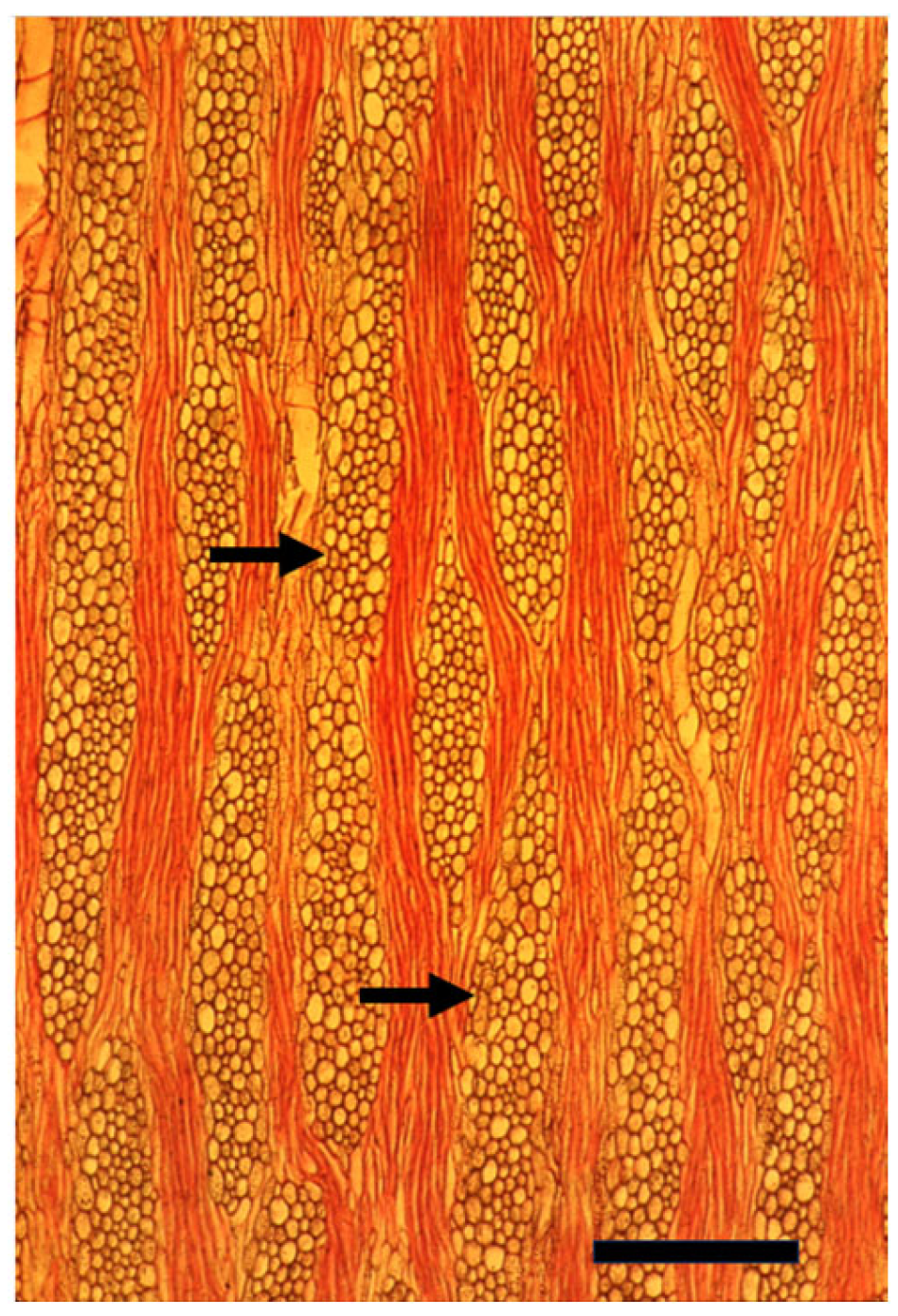
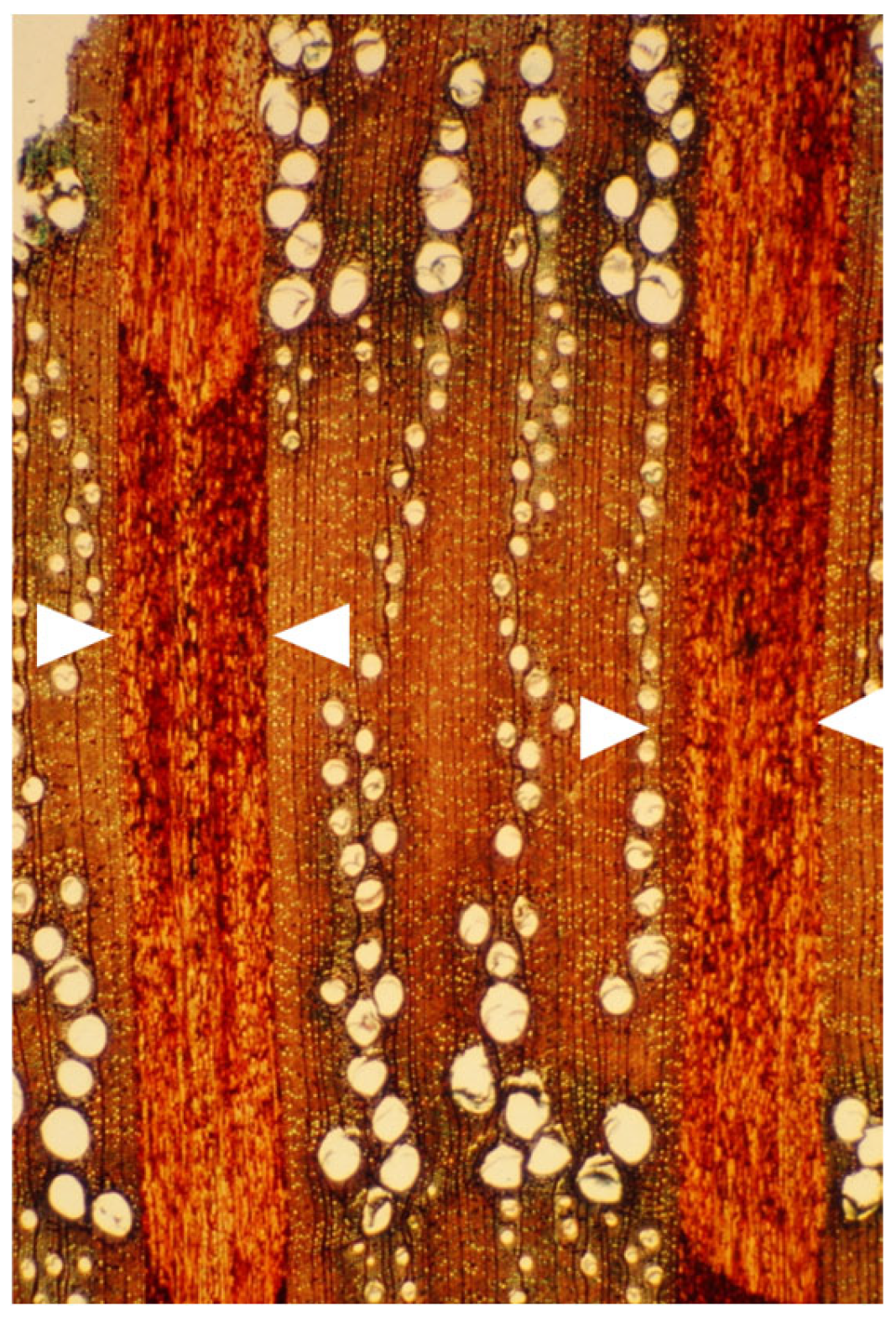
4. Cambial and Ray Structure
5. Signals That Regulate Cambial Biology
6. The Cambial Arena
7. The Recent Progress in Understanding Cambial Biology and Overlooking Cambial Ray Initials
8. Insufficient Enrichment of Ray Tissue While Sampling for Molecular Studies
9. Sample Mature Trunks
10. Stem Cells Are Always Differentiated
11. Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Crawley, M.J. Herbivory. In The Dynamics of Animal-Plant Interactions; Blackwell Scientific Publications: Oxford, UK, 1983. [Google Scholar]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant-herbivore interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Kishore, A.; Sumaila, U.R.; Issifu, I.; Hunter, B.P.; Belton, B.; Bush, S.R.; Cao, L.; Gelcich, S.; Gephart, J.A.; et al. Blue food demand across geographic and temporal scales. Nat. Commun. 2021, 12, 5413. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F. Economic Botany. A Textbook of Useful Plants and Plant Products; McGraw-Hill: New York, NY, USA, 1952. [Google Scholar]
- Williams, M. Deforesting the Earth. From Prehistory to Global Crisis; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Stroud, S.; Fennell, M.; Mitchley, J.; Lydon, S.; Peacock, J.; Bacon, K.L. The botanical education extinction and the fall of plant awareness. Ecol. Evol. 2022, 12, e9019. [Google Scholar] [CrossRef]
- Fahn, A. Plant Anatomy, 4th ed.; Pergamon Press: Oxford, UK, 1990. [Google Scholar]
- Poethig, R.S.; Sussex, I.M. The cellular parameters of leaf development in tobacco: A clonal analysis. Planta 1985, 165, 170–184. [Google Scholar] [CrossRef]
- Yu, K.M.J.; Oliver, J.; McKinley, B.; Weers, B.; Fabich, H.T.; Evetts, N.; Conradi, M.S.; Altobelli, S.A.; Marshall-Colon, A.; Mullet, J. Bioenergy sorghum stem growth regulation: Intercalary meristem localization, development, and gene regulatory network analysis. Plant J. 2022, 112, 476–492. [Google Scholar] [CrossRef]
- Tomlinson, P.B.; Takaso, T.; Cameron, E.K. Cone development in Libocedrus (Cupressaceae)—Phenological and morphological aspects. Am. J. Bot. 1993, 80, 649–659. [Google Scholar]
- Larson, P.R. The Vascular Cambium. Development and Structure; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Schweingruber, F.H.; Filartiga, A.L.; Jiří Doležal, J. Petioles of Terrestrial Plants; Kessel Publishing House: Remagen-Oberwinter, Germany, 2021. [Google Scholar]
- Liphschitz, N.; Waisel, Y. Sites of phellogen initiation. Bot. Gaz. 1975, 136, 146–150. [Google Scholar]
- Roth, I. Structural Patterns of Tropical Barks; Gebrüder Borntraeger: Berlin, Germany, 1981. [Google Scholar]
- Lev-Yadun, S.; Aloni, R. Experimental induction of dilatation meristem in Melia azedarach. Ann. Bot. 1992, 70, 379–386. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Aloni, R. Differentiation of the ray system in woody plants. Bot. Rev. 1995, 61, 45–88. [Google Scholar] [CrossRef]
- Carlquist, S. Comparative Wood Anatomy, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- IAWA Committee. IAWA list of microscopic features for hardwood identification. IAWA Bull. N. S. 1989, 10, 219–332. [Google Scholar]
- Carlquist, S. Xylem heterochrony: An unappreciated key to angiosperm origin and diversifications. Bot. J. Linn. Soc. 2009, 161, 26–65. [Google Scholar] [CrossRef]
- Carlquist, S. Wood and bark anatomy of Empetraceae; Comments on paedomorphosis in woods of certain small shrubs. Aliso 1989, 12, 497–515. [Google Scholar] [CrossRef]
- Bannan, M.W. The annual cycle of size changes in the fusiform cambial cells of Chamaecyparis and Thuja. Can. J. Bot. 1951, 29, 421–437. [Google Scholar] [CrossRef]
- Bannan, M.W. Ray contacts and rate of anticlinal division in fusiform cambial cells of some Pinaceae. Can. J. Bot. 1965, 43, 487–507. [Google Scholar] [CrossRef]
- Bannan, M.W.; Bayly, I.L. Cell size and survival in conifer cambium. Can. J. Bot. 1956, 34, 769–776. [Google Scholar] [CrossRef]
- Braun, H.J. Entwicklung und Bau der Holzstrahlen unter dem Aspect der Kontakt-Isolations-Differenzierung gegenuber dem Hydrosystem: I, Das Prinzip der Kontakt-Isolations-Differenzierung. Holzforschung 1967, 21, 33–37. [Google Scholar] [CrossRef]
- Braun, H.J.; Wolkinger, F.; Bohme, H. Entwicklung und Bau der Holzstrahlen unter dem Aspect der Kontakt-Isolations-Differenzierung gegenuber dem Hydrosystem: II, Die Typen der Kontakt-Holzstrahlen. Holzforschung 1967, 21, 145–153. [Google Scholar] [CrossRef]
- Braun, H.J.; Wolkinger, F.; Bohme, H. Entwicklung und Bau der Holzstrahlen unter dem Aspect der Kontakt-Isolations-Differenzierung gegenuber dem Hydrosystem: III, Die Typen der Kontakt-Isolations-Holzstrahlen und der Isolations-Holzstrahlen. Holzforschung 1968, 22, 53–60. [Google Scholar] [CrossRef]
- Braun, H.J.; Wolkinger, F.; Bohme, H. Entwicklung und Bau der Holzstrahlen unter dem Aspect der Kontakt-Isolations-Differenzierung gegenuber dem Hydrosystem: IV, Die Organisation der Holzstrahlen. Holzforschung 1968, 22, 153–157. [Google Scholar] [CrossRef]
- Rothwell, G.W.; Lev-Yadun, S. Evidence of polar auxin flow in 375 million-year-old fossil wood. Am. J. Bot. 2005, 92, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, G.W.; Sanders, H.; Wyatt, S.E.; Lev-Yadun, S. A fossil record for growth regulation: The role of auxin in wood evolution. Ann. Mo. Bot. Gard. 2008, 95, 121–134. [Google Scholar] [CrossRef]
- Tomescu, A.M.F.; Groover, A.T. Mosaic modularity: An updated perspective and research agenda for the evolution of vascular cambial growth. New Phytol. 2019, 222, 1719–1735. [Google Scholar] [CrossRef] [PubMed]
- Sachs, T. The control of patterned differentiation of vascular tissues. Adv. Bot. Res. 1981, 9, 151–262. [Google Scholar]
- Little, C.H.A.; Savidge, R.A. The role of plant growth regulators in forest tree cambial growth. Plant Growth Regul. 1987, 6, 137–169. [Google Scholar] [CrossRef]
- Kende, H.; Zeevaart, J.A.D. The five “classical” plant hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef]
- Arend, M.; Fromm, J. Concomitant analysis of cambial abscisic acid and cambial growth activity in poplar. Trees 2013, 27, 1271–1276. [Google Scholar] [CrossRef]
- Smetana, O.; Mäkilä, R.; Lyu, M.; Amiryousefi, A.; Rodríguez, F.S.; Wu, M.-F.; Solé-Gil, A.; Gavarrón, M.L.; Siligato, R.; Miyashima, S.; et al. High levels of auxin signalling define the stem-cell organizer of the vascular cambium. Nature 2019, 565, 485–489. [Google Scholar] [CrossRef]
- Buttò, V.; Deslauriers, A.; Rossi, S.; Rozenberg, P.; Shishov, V.; Morin, H. The role of plant hormones in tree-ring formation. Trees 2020, 34, 315–335. [Google Scholar] [CrossRef]
- Lev-Yadun, S. Radial fibres in aggregate rays of Quercus calliprinos Webb. —Evidence for radial signal flow. New Phytol. 1994, 128, 45–48. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Aloni, R. Vascular differentiation in branch junctions of trees: Circular patterns and functional significance. Trees Struct. Funct. 1990, 4, 49–54. [Google Scholar] [CrossRef]
- Behr, M.; Lutts, S.; Hausman, J.-F.; Guerriero, G. Jasmonic acid to boost secondary growth in hemp hypocotyl. Planta 2018, 248, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Sehr, E.M.; Agusti, J.; Lehner, R.; Farmer, E.E.; Schwarz, M.; Greb, T. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J. 2010, 63, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Agustia, J.; Herolda, S.; Schwarza, M.; Sancheza, P.; Ljungb, K.; Dunc, E.A.; Brewerc, P.B.; Beveridgec, C.A.; Siebererd, T.; Sehra, E.M. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20242–20247. [Google Scholar] [CrossRef]
- Hu, J.; Hu, X.; Yang, Y.; He, C.; Hu, J.; Wang, X. Strigolactone signaling regulates cambial activity through repression of WOX4 by transcription factor BES1. Plant Physiol. 2022, 188, 255–267. [Google Scholar] [CrossRef]
- Sorce, C.; Giovannelli, A.; Sebastiani, L.; Anfodillo, T. Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Rep. 2013, 32, 885–898. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, X.; Zhao, Y.; Zhang, M.; Wan, Y.; Cao, D.; Lu, S.; Lin, J. Genome-wide analysis reveals dynamic changes in expression of microRNAs during vascular cambium development in Chinese fir, Cunninghamia lanceolata. J. Exp. Bot. 2015, 66, 3041–3054. [Google Scholar] [CrossRef]
- Chen, B.; Chen, J.; Du, Q.; Zhou, D.; Wang, L.; Xie, J.; Li, Y.; Zhang, D. Genetic variants in microRNA biogenesis genes as novel indicators for secondary growth in Populus. New Phytol. 2018, 219, 1263–1282. [Google Scholar] [CrossRef]
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.P. The dynamics of cambial stem cell activity. Annu. Rev. Plant Biol. 2019, 70, 26.1–26.7. [Google Scholar] [CrossRef]
- Eswaran, G.; Zhang, X.; Rutten, J.P.; Han, J.; Iida, H.; Ortiz, J.L.; Mäkilä, R.; Wybouw, B.; Jiménez, B.P.; Vainio, L.; et al. Identification of cambium stem cell factors and their positioning mechanism. Science 2024, 386, 646–653. [Google Scholar] [CrossRef]
- Mattsson, J.; Sung, Z.R.; Berleth, T. Responses of plant vascular systems to auxin transport inhibition. Development 1999, 126, 2979–2991. [Google Scholar] [CrossRef] [PubMed]
- Forestan, C.; Farinati, S.; Varotto, S. The maize PIN gene family of auxin transporters. Front. Plant Sci. 2012, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-J.; Luo, J. The PIN-FORMED auxin efflux carriers in plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cheng, C.; Zhou, X.; Li, Y.; Hu, Y.; Yang, C.; Zhou, Y.; Soliman, T.M.A.; Zhang, H.; Wang, Q.; et al. Ethylene controls cambium stem cell activity via promoting local auxin biosynthesis. New Phytol. 2023, 239, 964–978. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Peng, S.; Han, H. Slow and rapid auxin responses in Arabidopsis. J. Exp. Bot. 2024, 75, 5471–5476. [Google Scholar] [CrossRef]
- Cohen, I.; Efroni, I. Mobile signals, patterning, and positional information in root development. Plant Physiol. 2024, 196, 2175–2183. [Google Scholar] [CrossRef]
- Wilson, J.W.; Grange, R.I. Regeneration of tissues in wounded stems: A quantitative study. Ann. Bot. 1984, 53, 515–525. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Growth and Development of Trees; Academic Press: New York, NY, USA, 1971; Volume II. [Google Scholar]
- Kucukoglu, M.; Chaabouni, S.; Zheng, B.; Mähönen, A.P.; Helariutta, Y.; Nilsson, O. Peptide encoding Populus CLV3/ESR-RELATED 47 (PttCLE47) promotes cambial development and secondary xylem formation in hybrid aspen. New Phytol. 2020, 226, 75–852. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Chen, W.; Xu, M.; Zhou, R.; Shou, H.; Chen, J. High resolution anatomical and spatial transcriptome analyses reveal two types of meristematic cell pools within the secondary vascular tissue of popular stem. Mol. Plant 2023, 16, 809–828. [Google Scholar] [CrossRef]
- Wybouw, B.; Zhang, X.; Mähönen, A.P. Vascular cambium stem cells: Past, present and future. New Phytol. 2024, 243, 851–865. [Google Scholar] [CrossRef]
- Lev-Yadun, S. Induction of sclereid differentiation in the pith of Arabidopsis thaliana (L.) Heynh. J. Exp. Bot. 1994, 45, 1845–1849. [Google Scholar] [CrossRef]
- Lev-Yadun, S. Circular vessels in the secondary xylem of Arabidopsis thaliana (L.) Heynh. (Brassicaceae). IAWA J. 1996, 17, 31–35. [Google Scholar] [CrossRef]
- Lev-Yadun, S. Fibres and fibre-sclereids in wild-type Arabidopsis thaliana. Ann. Bot. 1997, 80, 125–129. [Google Scholar] [CrossRef]
- Zobel, B.J.; van Buijtenen, J.P. Wood Variation. Its Causes and Control; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Schweingruber, F.H. Anatomy of European Woods; Verlag Paul Haupt: Bern, Switzerland, 1990. [Google Scholar]
- Larisch, C.; Dittrich, M.; Wildhagen, H.; Lautner, S.; Fromm, J.; Polle, A.; Hedrich, R.; Rennenberg, H.; Müller, T.; Ache, P. Poplar wood rays are involved in seasonal remodeling of tree physiology. Plant Physiol. 2012, 160, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.C.; Ferro, A.; Capote, T.; Usié, A.; Correia, B.; Pinto, G.; Menéndez, E.; Marum, L. Laser microdissection of woody and suberized plant tissues for RNA-seq analysis. Mol. Biotechnol. 2023, 65, 419–432. [Google Scholar] [CrossRef]
- Namvar, S.; Boucher, É.; Deslauriers, A.; Morin, H.; Savard, M.M. Monitoring weekly δ13C variations along the cambium–xylem continuum in the Canadian eastern boreal forest. Tree Physiol. 2024, 44, tpae136. [Google Scholar] [CrossRef]
- Uggla, C.; Moritz, T.; Sandberg, G.; Sundberg, B. Auxin as a positional signal in pattern formation in plants. Proc. Natl. Acad. Sci. USA 1996, 93, 9282–9286. [Google Scholar] [CrossRef]
- Dinwoodie, J.M. Tracheid and fibre length in timber: A review of literature. Forestry 1961, 34, 125–144. [Google Scholar] [CrossRef]
- Lachenbruch, B.; Moore, J.R.; Evans, R. Radial variation in wood structure and function in woody plants, and hypotheses for its occurrence. In Size- and Age-Related Changes in Tree Structure and Formation, Tree Physiology; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 4, pp. 121–164. [Google Scholar] [CrossRef]
- Petit, G. An appreciation of apex-to-base variation in xylem traits will lead to more precise understanding of xylem phenotypic plasticity. New Phytol. 2024, 244, 1175–1180. [Google Scholar] [CrossRef]
- Lev-Yadun, S. Stem cells in plants are differentiated too. Curr. Top. Plant Biol. 2003, 4, 93–102. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lev-Yadun, S. Regulating the Vascular Cambium: Do Not Forget the Vascular Ray Initials and Their Derivatives. Plants 2025, 14, 971. https://doi.org/10.3390/plants14060971
Lev-Yadun S. Regulating the Vascular Cambium: Do Not Forget the Vascular Ray Initials and Their Derivatives. Plants. 2025; 14(6):971. https://doi.org/10.3390/plants14060971
Chicago/Turabian StyleLev-Yadun, Simcha. 2025. "Regulating the Vascular Cambium: Do Not Forget the Vascular Ray Initials and Their Derivatives" Plants 14, no. 6: 971. https://doi.org/10.3390/plants14060971
APA StyleLev-Yadun, S. (2025). Regulating the Vascular Cambium: Do Not Forget the Vascular Ray Initials and Their Derivatives. Plants, 14(6), 971. https://doi.org/10.3390/plants14060971





