Plant-Derived Monoterpene Therapies in Parkinson’s Disease Models: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
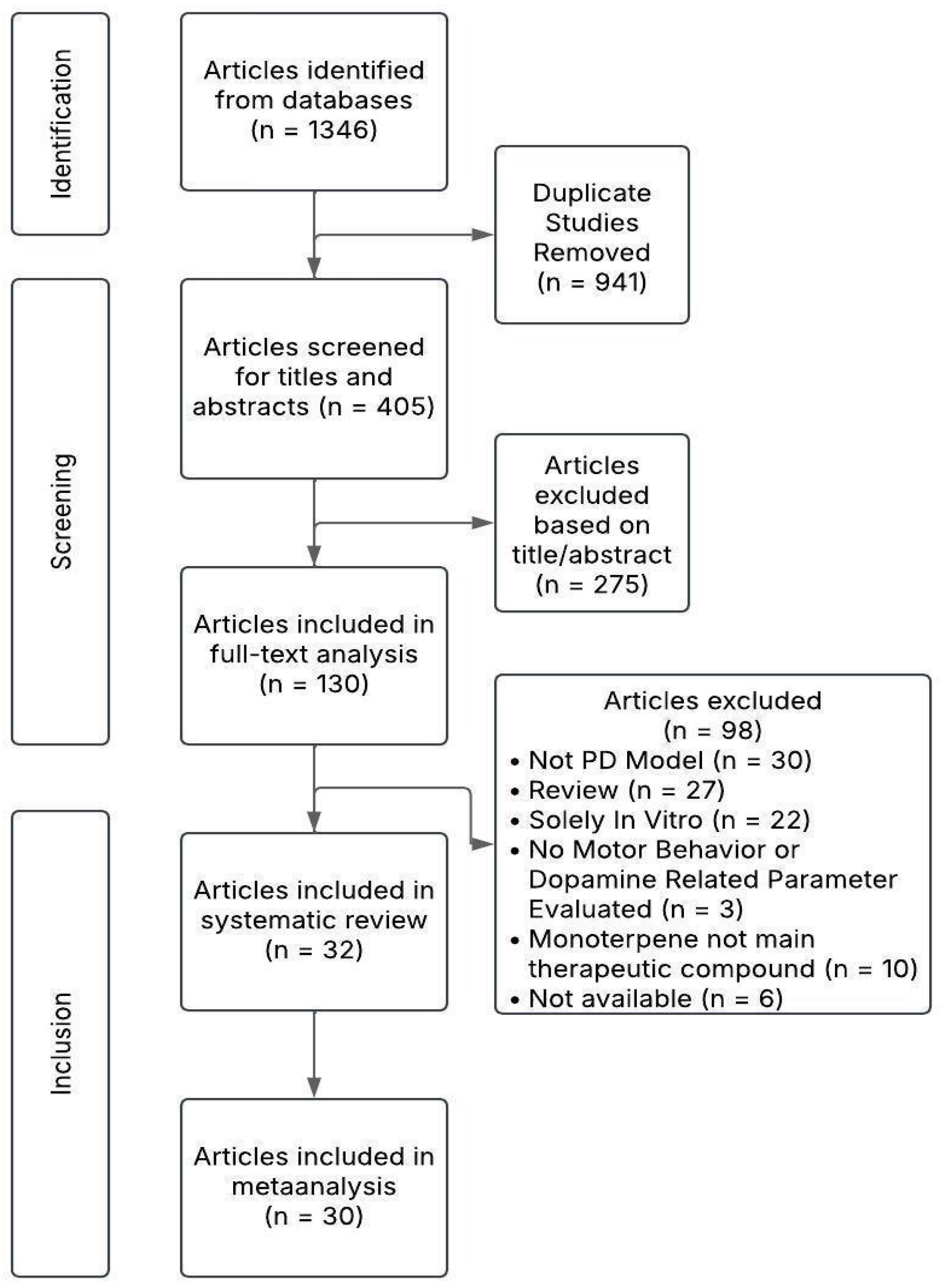
2.1. Search Results
2.2. Characteristics of Publications
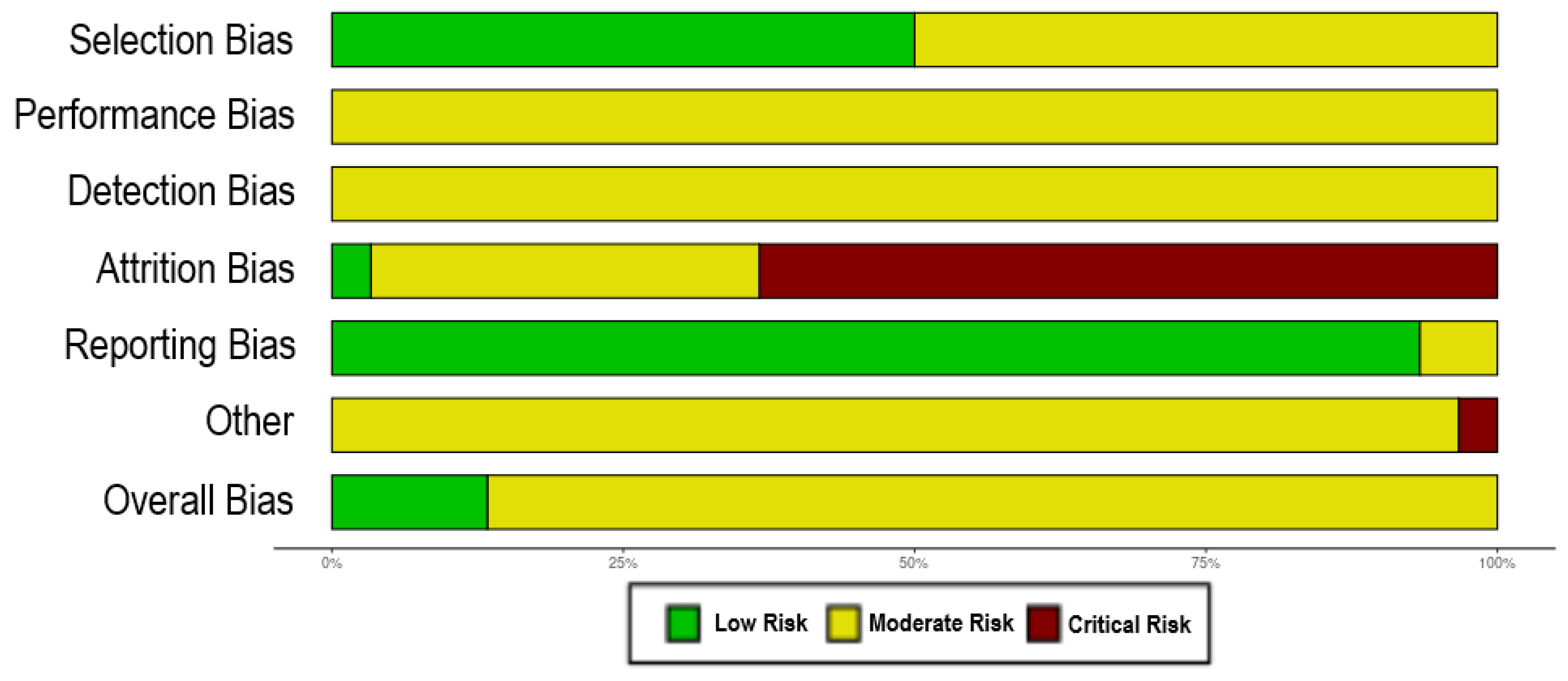
2.3. Risk of Bias
| Monoterpene Therapy | Animal Model Characteristics | Motor Behavior | Dopamine- Related Parameters | Antioxidant Properties | Inflammatory Parameters | Other Findings | Reference |
|---|---|---|---|---|---|---|---|
| Borneol (i.g. for 30 days before lesion. 7.5 mg/kg; 15 mg/kg; 20 mg/kg; 30 mg/kg) | Male C57BL/6 Mouse; 7 weeks old. MPTP i.p. 20 mg/kg for 30 days | ↑ Distance traveled in Open Field ↑ Performance in Pole Climbing ↑ Time in Suspension Test | SN: ↑ TH positive cells, ↑ DAT positive cells. STR: ↑ TH positive fibers, ↑ DAT positive fibers, ↑ DAT protein and mRNA content, ↑ VMAT2 protein and mRNA content, ↑ DA, ↑ DOPAC, ↑ HVA | SN: ↓ MDA, ↑ SOD, ↑ GSH-px | SN: ↓ IL-6, ↓ IL-1β, ↓ TNF-α | ↓ Freezing in Open Field | Ding et al., 2024 [27] |
| Carvacrol (i.p. for 14 days after lesion. 10 mg/kg) | Male Sprague-Dawley Rat; 200–250 g. 6-OHDA (12 μg, intrastriatal, unilateral) | SN: ↑ TH positive cells | SN: ↓ GFAP positive cells | SN: ↑ TRPC positive DA neurons. ↓ TRPC positive astrocytes. ↑ TRPA1 mRNA and protein level | Akan et al., 2023 [28] | ||
| Carvacrol (i.p. for 3 days before lesion. 10 mg/kg) | Male Wistar Rat; 250–300 g. 6-OHDA (12,5 μg, intrastriatal, unilateral) | ↓ Apomorphine induced rotations | SN: ↓ MDA, ↓ NO2-, ↑ Catalase Activity | Baluchnejadmojarad et al., 2014 [29] | |||
| Carvacrol (i.p. same day as lesion. 40 mg/kg) | Male C57BL/6 Mouse; 3 months old. 6-OHDA (6 μg, intrastriatal, unilateral) | ↓ Ipsilateral forelimb use in Cylinder Test | SN: ↑ TH protein CPU: ↑ TH protein | SN: ↓ Caspase 3 protein CPU: ↓ Caspase 3 protein | Dati et al., 2017 [30] | ||
| Carvacrol (i.p. for 49 days [7 before and 42 after lesion]. 25 mg/kg, 50 mg/kg, 100 mg/kg) | Male Wistar Rat; 250–350 g. 6-OHDA (16 μg, intra-MFB, unilateral) | ≈ Apomorphine induced rotations | CPU: ≈ MDA, ≈ Thiol | ↑ Performance in Passive Avoidance Test ≈ Tail Flick Latency | Haddadi et al., 2018 [31] | ||
| Carvacrol (i.p. for 49 days [7 before and 42 after lesion]. 25 mg/kg) | Male Wistar Rat; 250–300 g. 6-OHDA (16 μg, intra-MFB, unilateral) | ≈ Apomorphine induced rotations (↓ with physical exercise) | CPU: ≈ MDA (↓ with physical exercise), ≈ Thiol (↑ with physical exercise) Hipp: ≈ MDA (↓ with physical exercise), ≈ Thiol | ↑ Performance in Passive Avoidance Test | Hamzehloei et al., 2019 [32] | ||
| Carvacrol (i.p. for 30 days along with lesion. 12.5 mg/kg, 25 mg/kg) | Male Wistar Rat; 7 months old. Reserpine s.c. 0.1 mg/kg for 30 days every other day | ↓ Catalepsy ≈ Distance traveled in Open Field ↓ Vacuous Chewing Movements ≈ Rearing in Open Field | SN: ↑ TH positive cells CPU: ↑ TH optic density | Lins et al., 2018 [33] | |||
| Carvacrol (i.p. for 15 days after lesion. 10 mg/kg, 15 mg/kg, 20 mg/kg) | Male Wistar Rat; 200–250 g. 6-OHDA (16 μg, intrastriatal, unilateral) | ↓ Apomorphine induced rotations ↓ Catalepsy ↓ Inversion and Total time in Pole Test ↓ Inversion and Total time in Beam Walking Test ↑ Time to fall from Rotarod ↑ Distance traveled in Rotarod ≈ Distance traveled in Open Field | CPU: ↑ GSH, ↓ MDA | Manouchehrabadi et al., 2020 [34] | |||
| Carvacrol (p.o. for 15 days before lesion. 25 μg/kg) | Male Wistar Rat; 60 days old. 6-OHDA (10 μg, intra-SN, unilateral) | SN: ↑ TH positive signal | ↓ CSF IL-1β ↓ Serum IL-1β ↓ Serum TNF-α | Normal blood cell count and serum aminotransferases activity | Ribeiro et al., 2019 [35] | ||
| Carvacrol (p.o. for 21 days along with lesion. 25 mg/kg, 50 mg/kg, 100 mg/kg) | Male Swiss Mouse; 10–12 weeks old. Rotenone i.p. 1.5 mg/kg for 21 days. | ↑ Time to fall from Rotarod ↑ Distance traveled and maximum speed in Open Field ↓ Freezing time and immobility time in Open Field | SN: ↑ TH positive signal and protein | SN: ↑ NRF2 protein, ↑ NQO-1 protein, ↑ HO-1 protein | SN: ↓ NLRP3 protein, ↓ ASC protein, ↓ TNF-α, ≈ IL-6, ↓ IL-1β, ↑ IL-4 | ↓ α-synuclein in SN | Shah et al., 2024 [36] |
| Citronellol (p.o. for 28 days along with lesion. 25 mg/kg) | Male Wistar Rat; 280–300 g. Rotenone i.p. 2.5 mg/kg for 28 days. | SN: ↑ TH positive cells CPU: ↑ TH positive fibers | SN: ↑ Catalase, ↑ GSH, ↑ Nrf2, ↓ MDA, ↑ SOD | SN: ↓ IL-1β, ↓ IL-6, ↓ MMP-9, ↓ TNF-α, ≈ COX-2, ↓ iNOS CPU: ↓ IBA1, ↓ GFAP | ↓ α-synuclein in SN ↓ Bax, ≈ Bcl-2, ≈ mTOR in SN ↓ LC-3, ≈ p62 in SN | Jayaraj et al., 2022 [37] | |
| Geraniol (p.o. for 7 days before and along with lesion. 100 mg/kg) | Male C57BL/6 Mouse; 25–30 g. MPTP i.p. 30 mg/kg for 4 days. | ↑ Time to fall from Rotarod ↑ Number of Steps in Drag Test ↑ Fore Paw Stride Length in Foot Print Test | CPU: ↑ DA, ↑ DOPAC, ↑ HVA, ↑ TH protein, ↑ DAT protein, ↑ VMAT-2 protein SN: ↑ TH positive cells | CPU: ↑ MDA, ↑ GSH | ↑ BDNF (protein and mRNA), ↑ GDNF (protein and mRNA) in CPU | Rekha et al., 2013 [38] | |
| Geraniol (p.o. for 7 days along with lesion. 50 mg/kg, 100 mg/kg, 200 mg/kg) | Male C57BL/6 Mouse; 25–30 g. MPTP i.p. 30 mg/kg for 7 days. | ↑ Hanging Time in Hang Test | CPU: ↓ MAO-B activity, ↑ TH positive fibers SN: ↑ DA, ↑ DOPAC, ↑ HVA | ↓ α-synuclein mRNA and protein in SN and CPU | Rekha et al., 2013 [39] | ||
| Geraniol (p.o. 10 doses along with lesion through 35 days. 100 mg/kg) | Male C57BL/6 Mouse; 25–30 g. 10 doses of MPTP 25 mg/kg s.c. with probenecid 250 mg/kg i.p. through 35 days. | ↓ Bradykinesia in Pole Test ↑ Distance traveled in Open Field ↓ Catatonia ↑ Rearing in Open Field | SN: ↑ DAT positive cells | CPU: ↑ GSH-Px activity, ↓ SOD activity, ↓ Catalase activity | ↑ Grooming in Open Field ↑ Bcl-2 mRNA and protein, ↓ Bax mRNA and protein, ↓ Cyc1 mRNA and protein, ↓ Caspase-9 mRNA and protein in SN ↑ Bcl-2 protein, ↓ Bax protein, ↓ Cyc1 protein and ↓ Caspase-9 protein in CPU | Rekha and Selvakumar, 2014 [40] | |
| Limonene (i.p. 5 days a week for 4 weeks along with lesion. 50 mg/kg) | Male Wistar Rat; 260–300 g. Rotenone i.p. 2.5 mg/kg 5 days a week for 4 weeks. | ↑ Time to fall from Rotarod | SN: ↑ TH positive cells CPU: ↑ TH positive fibers | SN: ↓ MDA, ↑ SOD activity, ↑ Catalase, ↑ GSH | SN: ↓ TNF-α, ↓ IL-6, ↓ IL-1β CPU: ↓ GFAP positive cells, ↓ IBA1 positive cells, ↓ iNOS, ↓ COX-2, ↓ p-NFκB, ↓ p-IκB | ↓ α-synuclein in CPU ↑ BDNF in CPU ↓ Phosphorylation of P38 and JNK in CPU ↑ Phosphorylation of mTOR in CPU ↑ Mitochondrial Complex 1 in CPU ↓ Bax, ↑ Bcl2, ↓ Cl-Caspase 3, ↓ Cl-Caspase 9, ↓ Cytochrome-C, ↓ CHOP and ↓ p-MST1 in CPU | Eddin et al., 2023 [41] |
| Linalool (p.o. for 15 days after lesion. 25 mg/kg, 50 mg/kg, 100 mg/kg) | Male Wistar Rat; 250–280 g. 6-OHDA (12 μg, intrastriatal, unilateral) | ↓ Apomorphine induced rotations ↑ Distance traveled in Open Field | CPU: ↑ DA, ↑ DOPAC, ≈ HVA, ↑ TH positive fibers, ↑ DAT positive fibers | ↓ Nitrites and ↓ MDA in CPU, Hipp, and PFC | de Lucena et al., 2020 [42] | ||
| Linalool (p.o. for 7 days before lesion. 12.5 mg/kg, 25 mg/kg). | Male C57BL/6 Mouse; 20–25 g. MPTP 4 i.p. injections. 20 mg/kg | ↑ Grip Strength ↑ Latency to Fall in Wire Hang Test ↑ Distance traveled in Open Field ↑ Mean velocity in Open Field | SN: ↑ TH positive area | ↑ Time in Central Zone in Open Field ↓ Immobility Time and ↑ Swimming Time in Forced Swimming Test ↑ Time spent in open arms, ↑ Open arm entries and ↓ Time spent in closed arms in Plus-Maze test | Chang et al., 2024 [43] | ||
| Menthol (p.o. for 28 days after lesion. 10 mg/kg, 20 mg/kg). | Male Wistar Rat; 280–320 g. LPS (intra-SN, unilateral) | ↓ Apomorphine induced rotations | SN: ↑ TH positive cells and protein | SN: ↓ IBA1 positive cells and protein, ↓ iNOS protein and mRNA, ↓ COX-2 protein and mRNA, ↓ IL-1β mRNA, ↓ IL-6 mRNA, ↓ TNF-α mRNA | Du et al., 2020 [44] | ||
| Myrcene (p.o. 5 days a week for 4 weeks along with lesion. 50 mg/kg) | Male Wistar Rat; 280–300 g. Rotenone i.p. 2.5 mg/kg 5 days a week for 4 weeks. | SN: ↑ TH positive cells CPU: ↑ TH positive fibers | SN: ↓ MDA, ↑ GSH, ↑ Catalase, ↑ SOD activity | SN: ↓ TNF-α, ↓ IL-6, ↓ IL-1β, ↓ MMP-9, ↓ iNOS, ↓ COX-2 CPU: ↓ IBA1 positive cells, ↓ GFAP positive cells | SN: ↓ Bax, ↑ Bcl-2, ↓ Bax/Bcl-2 ratio CPU: ↓ α-synuclein, ↓ Beclin-1, ↓ LC3B, ↓ P62, ↑ mTOR phosphorylation | Azimullah et al., 2023 [45] | |
| Myrtenal (i.p. for 5 days before lesion. 50 mg/kg) | Male Wistar Rat; 250–300 g. 6-OHDA (10 μg, intrastriatal, unilateral) | ↓ Apomorphine induced rotations ↓ Number of falls in Rotarod | ↑ DA in whole brain | ↓ MDA in ipsilateral side of brain, ≈ GSH, ≈ SOD, ↑ Catalase in whole brain, ≈ GSH-Px | Slight body weight loss after pre-treatment. ↑ Step-through Latency in Passive Avoidance Test | Tancheva et al., 2020 [46] | |
| Myrtenol (p.o. for 28 days along with lesion. 5 mg/kg) | Male Swiss Mouse; 45–65 g. Reserpine s.c. 0.1 mg/kg every two days for 28 days | Delayed catalepsy ↓ Vacuous Chewing Movements ≈ Distance traveled and average speed in Open Field | ≈ TH+ positive cells in SN and VTA ↑ TH+ positive fibers in CPU | PFC: ↓ MDA, ≈ Total Antioxidant Status, Total Oxidant Status and Oxidative Stress Index Hipp: ≈ MDA, Total Antioxidant Status, Total Oxidant Status and Oxidative Stress Index CPU: ≈ MDA and Total Antioxidant Status, ↓ Total Oxidant Status and Oxidative Stress Index | ↑ Olfactory discrimination ↑ Novel Object Recognition ≈ Time in Open Arms in Plus-Maze Test ≈ Central Zone Entries in Open Field | Silva-Martins et al., 2021 [47] | |
| Perillyl Alcohol (p.o. for 14 days [7 before and 7 after lesion]. 100 mg/kg) | Male Wistar Rat; 250–300 g. 6-OHDA (16 μg, intrastriatal, unilateral) | ↓ Motor Asymmetry ↓ Escape Latency in Narrow Beam Test ≈ Time to Cross in Narrow Beam Test ↑ Rearing | CPU: ↑ TH mRNA and protein | CPU: ↓ Intracellular ROS Generation, ↑ Nrf2 protein | CPU: ↓ IL-1β mRNA, ↓ TNF-α mRNA | CPU: ↓ DNA Fragmentation, ↓ Caspase 3 Activation, ↓ Bax mRNA, ↑ Bax protein, ↑ Bcl-2 mRNA and protein, ↑ PGC-1α mRNA and protein, ↑ Drp1 protein | Anis et al., 2020 [48] |
| α-pinene (nanoemulsion p.o. for 6 days after lesion). 50 mg/kg, 100 mg/kg. | Male Wistar Rat; 200–220 g. Reserpine 5 mg/kg i.p. single dose. | ↓ Tremulous Jaw Movements ↑ Rearing ↓ Catalepsy ↓ Hind Limb Rigidity | Whole Brain: ↓ MDA, ↑ SOD Activity, ↑ Catalase Activity, ↑ Glutathione, ↓ NO Level | ↑ Grooming Behavior | Srivastava et al., 2021 [49] | ||
| α-pinene (nanoemulsion p.o. for 15 days before lesion). 50 mg/kg, 100 mg/kg. | Male Wistar Rat; 200–220 g. Haloperidol 1 mg/kg i.p. single dose | ↓ Catalepsy ↑ Swimming Performance ↑ Locomotor Performance ↓ Akinesia ↑ Time to fall from Rotarod | Srivastava et al., 2021 [49] | ||||
| α-pinene (nanoemulsion p.o. for 28 days along with lesion [7 days a week]). 50 mg/kg, 100 mg/kg) | Male Wistar Rat; 200–220 g. Rotenone 1.5 mg/kg s.c. for 28 days. | ↑ Time to fall from Rotarod ↑ Time to fall from Hanging Wire ↑ Locomotor Activity ↑ Postural Stability ↑ Step Alternation ↑ Forelimb Locomotion | Whole Brain: ↓ MDA, ↑ SOD Activity, ↑ Catalase Activity, ↑ Glutathione, ↓ NO Level | Srivastava et al., 2024 [50] | |||
| α-pinene (nanoemulsion p.o. for 28 days along with lesion [5 days a week in the last 28 days]). 50 mg/kg, 100 mg/kg) | Male Wistar Rat; 200–220 g. Trichloroethylene 1000 mg/kg p.o. for 8 weeks (5 days a week). | ↑ Time to fall from Rotarod ↑ Time to fall from Hanging Wire ↑ Locomotor Activity ↑ Postural Stability ↑ Step Alternation ↑ Forelimb Locomotion | Whole Brain: ↓ MDA, ↑ SOD Activity, ↑ Catalase Activity, ↑ Glutathione, ↓ NO Level | Srivastava et al., 2024 [50] | |||
| Safranal (i.p. for 14 days. 0.1 mg/kg, 0.2 mg/kg, 0.4 mg/kg) | Male C57BL/6 Mouse. MPTP 25 mg/kg i.p. for 5 days. | ↓ Catalepsy ↑ Grip Strength ↑ Distance traveled in Open Field ↑ Performance in Pole Climbing ↑ Time to fall from Rotarod ↑ Swing speed and stride length of hindlimbs and forelimbs | CPU: ↑ TH protein, mRNA and fluorescence intensity, ↑ DA | SN: ↓ NLRP3 mRNA and protein, ↓ IL-1β mRNA and protein | SN: ↓ Caspase-1 mRNA and protein | Yang et al., 2024 [51] | |
| Thymol (i.p. for 28 days along with lesion. 50 mg/kg) | Male Wistar Rat; 280–300 g. Rotenone 2.5 mg/kg i.p. for 28 days. | SN: ↑ TH positive cells CPU: ↑ TH positive fibers | SN: ↓ MDA, ↑ GSH, ↑ SOD Activity, ↑ Catalase Activity | CPU: ↓ GFAP positive cells, ↓ Iba-1 positive cells, ↓ COX-2 protein, ↓ iNOS protein SN: ↓ IL-1β, ↓ IL-6, ↓ TNF-α | Javed et al., 2019 [52] | ||
| Thymol (i.p. for 15 days after lesion. 20 mg/kg, 30 mg/kg, 40 mg/kg) | Male Wistar Rat; 200–250 g. 6-OHDA (15 μg, intrastriatal, unilateral) | ↓ Apomorphine induced rotations ↓ Catalepsy ↓ Inversion and Total time in Pole Test ↓ Inversion and Total time in Beam Walking Test ≈ Time to fall from Rotarod ↑ Distance traveled in Rotarod ↑ Distance traveled in Open Field | CPU: ↑ GSH, ↓ MDA | Nourmohammadi et al., 2022 [53] | |||
| Thymol (p.o. for 35 days along with lesion. 30 m/kg) | Male Sprague-Dawley Rat; 300–320 g. Mn2+ 10 mg/kg i.p. for 35 days | ↓ Latency to move in Open Field ↑ Distance traveled in Open Field ↑ Rearing in Open Field ↓ Latency to move in Swimming Test ↓ Swimming Time ↑ Swimming Direction Score ↓ Catalepsy | ↑ DA in whole brain | ↑ Nrf2 in whole brain ↑ HO-1 in whole brain ↑ Total Antioxidant Capacity in whole brain ↑ SOD in whole brain ↓ MDA in whole brain | ↓ COX-2 in whole brain ↓ iNOS in whole brain ↓ TNF-α in whole brain ↓ TLR4 in whole brain ↓ NLRP3 in whole brain ↓ NFκB in whole brain ↓ IL-1β in whole brain ↓ GFAP mRNA in whole brain ↓ Aif mRNA in whole brain | ↑ Grooming in Open Field ↑ Spontaneous Alternation in Y-Maze Test ↑ NE, 5-HT and GABA in whole brain ↓ ACHE activity in whole brain ↑ BDNF in whole brain ↓ Glutamate in whole brain ↓ Caspase 1, Caspase 3 and BAX mRNA in whole brain ↑ Bcl2 mRNA in whole brain ↓ GSK-3β mRNA in whole brain ≈ Histopathological alterations in cerebral cortex ↓ Histopathological alterations in Hipp and CPU | Abu-Elfotuh et al., 2022 [54] |
| Thymoquinone (p.o. for 2 days before lesion. 5 mg/kg, 10 mg/kg) | Male Wistar Rat; 190–250 g. 6-OHDA (12.5 μg, intrastriatal, unilateral) | ↓ Apomorphine induced rotations | SN: ↓ MDA, ≈ Nitrites, ≈ SOD | ↑ Nissl stained neurons in SN | Sedaghat et al., 2014 [55] | ||
| Thymoquinone (p.o. every 2 days for 30 days along with lesion. 7.5 mg/kg, 15 mg/kg) | Male Wistar Rat; 250–350 g. Rotenone 1 mg/kg s.c. every 2 days for 30 days. | ≈ Time to fall from Rotarod ≈ Rearing in Open Field ≈ Catalepsy | SN: ↑ DA, ↑ TH positive cells CPU: ↑ TH positive fibers | ↓ Serum pro-oxidant/antioxidant balance | ≈ Body Weight ≈ Parkin in SN and CPU ↓ Drp1 in SN and CPU | Ebrahimi et al., 2017 [56] | |
| Thymoquinone (i.p. for 1 week along with lesion. 10 mg/kg) | Male C57BL/6 Mouse; 25–30 g. MPTP 25 mg/kg i.p. for 5 days | SN: ↑ TH positive cells CPU: ↑ DAT positive fibers | CPU: ↑ SOD Activity, ↑ Catalase Activity, ↓ MDA, ↑ GSH | CPU: ↓ IL-1β, ↓ IL-6, ↓ TNF-α, ↓ COX-2 protein, ↓ iNOS protein | Ardah et al., 2019 [57] | ||
| Thymoquinone (i.p. for 7 days before and along with lesion. 10 mg/kg) | C57BL/6 Mouse; 25–30 g. MPTP 25 mg/kg i.p. for 5 days | SN: ↑ TH positive cells | SN: ↓ MDA, ↑ SOD Activity, ↑ GSH-Px Activity, ↓ 8OH-dG, ↑ Nrf2, ↑ HO-1, ↑ NQO-1, ↑ GST | SN: ↓ α-synuclein, ↓ Fluoro Jade staining | Dong et al., 2021 [58] |
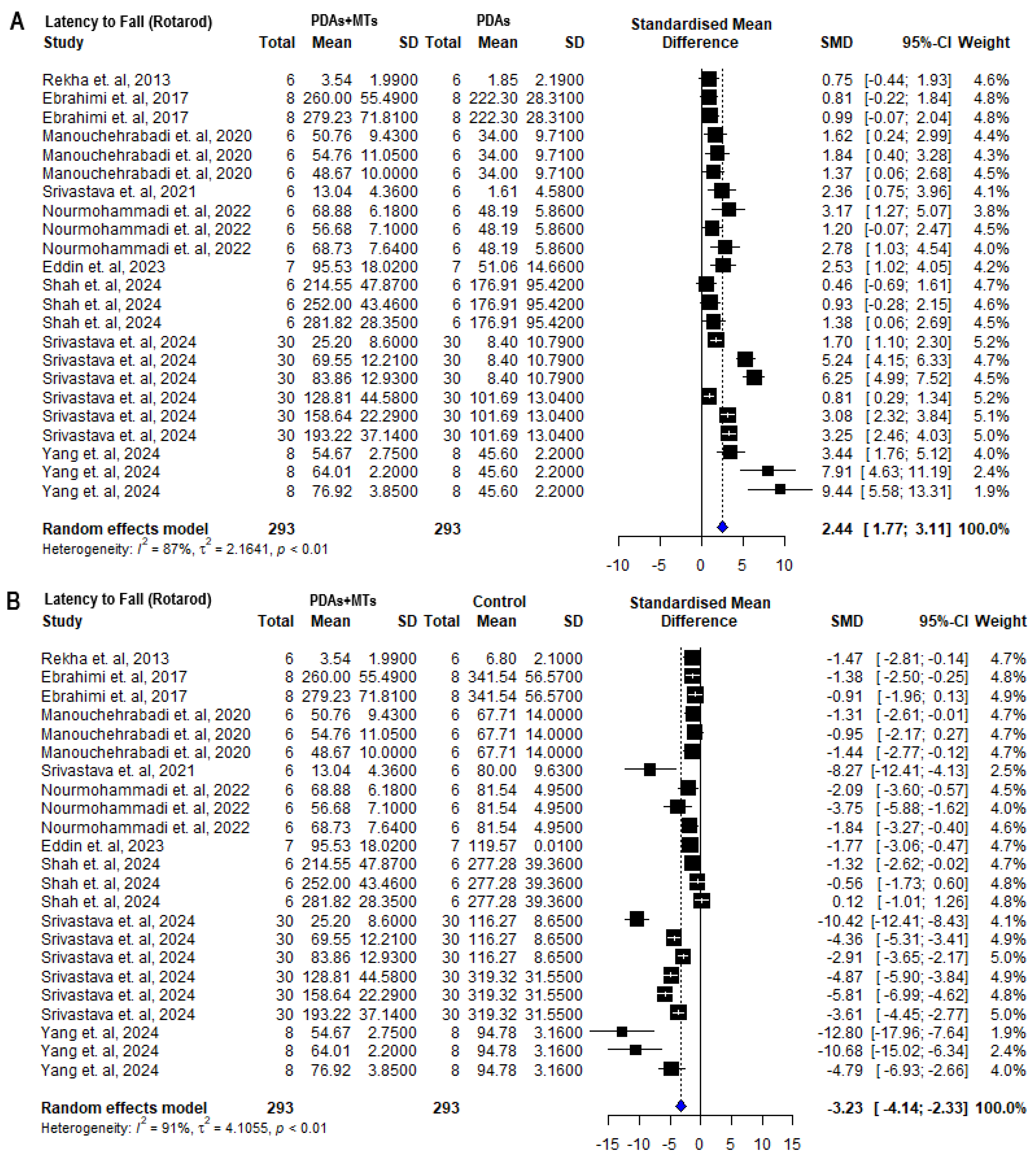
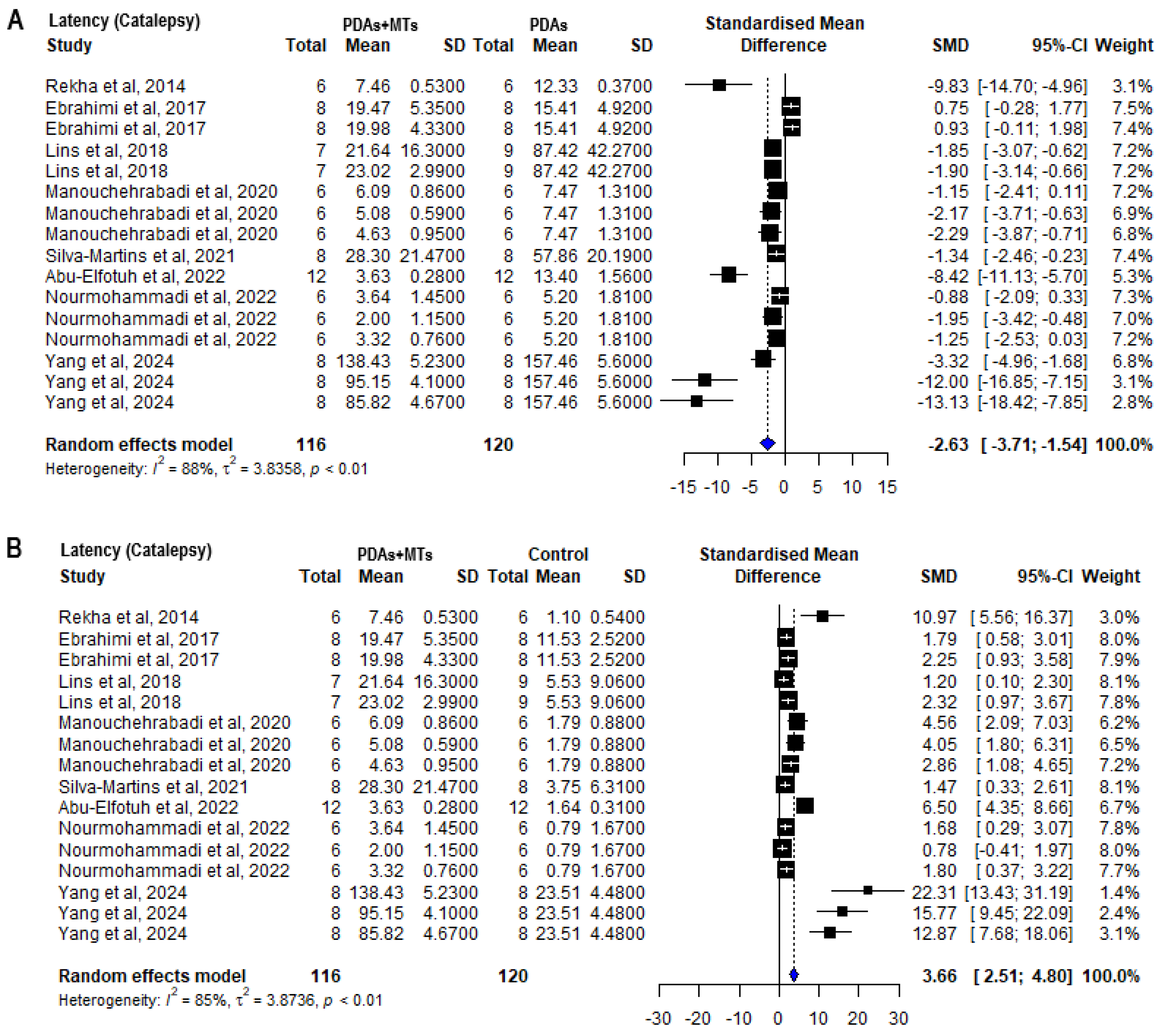
2.4. Effects of MTS in Motor Behavioral Parameters
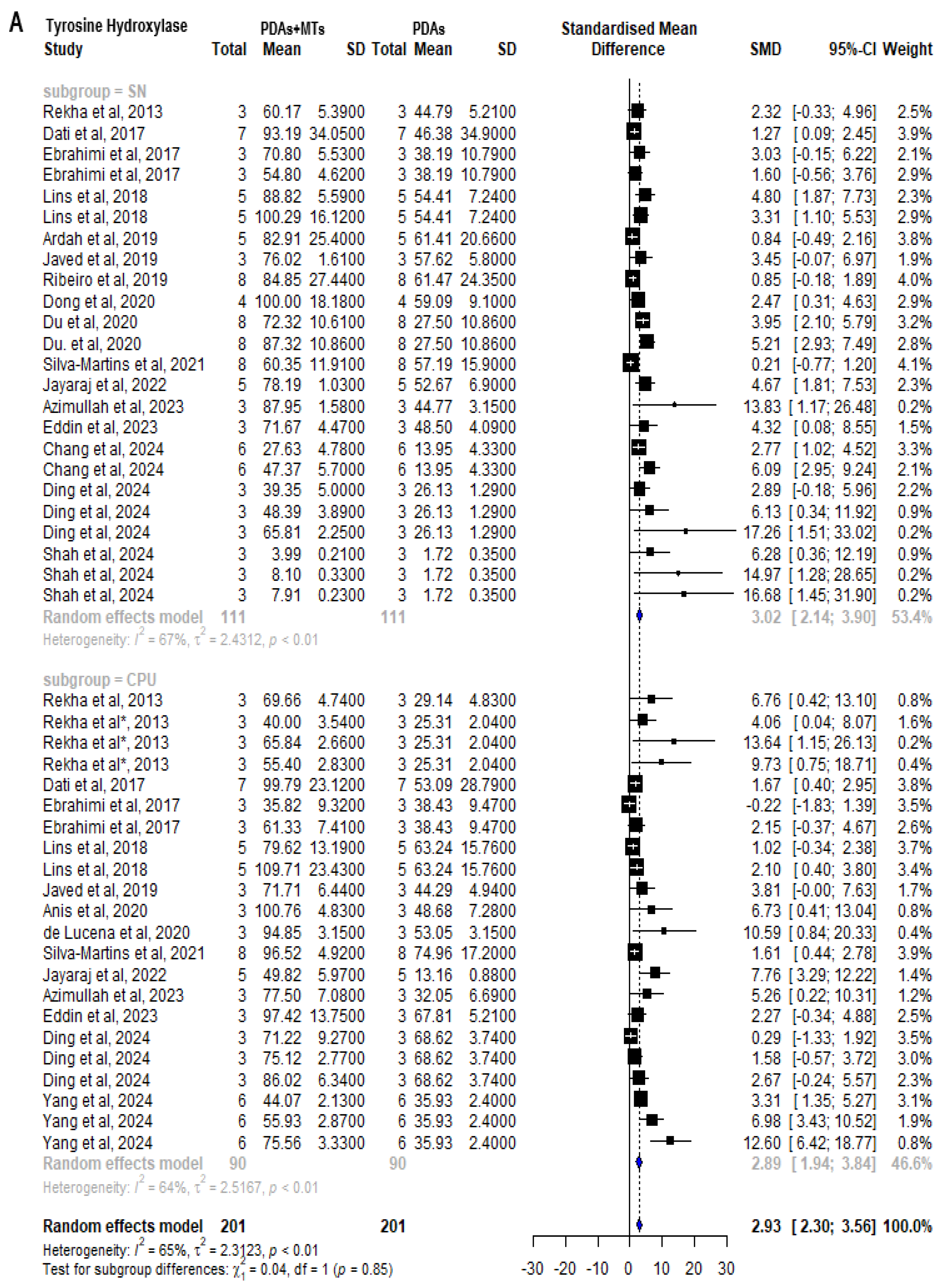
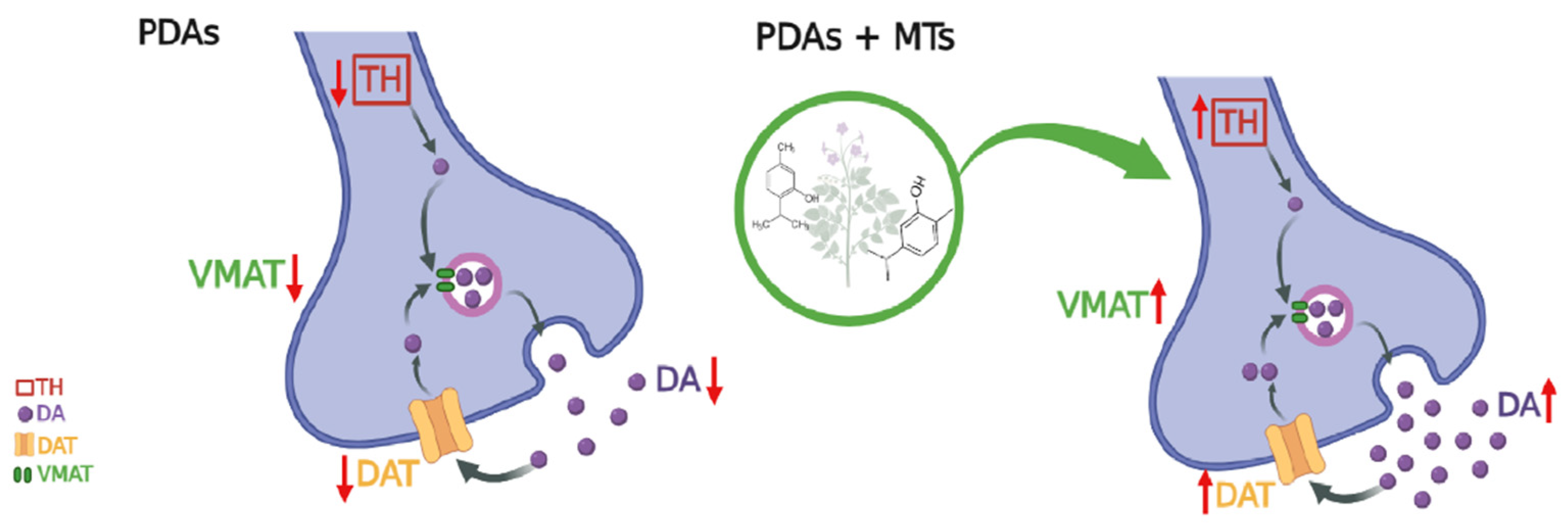
2.5. Effect of MTs on Dopamine-Related Parameters
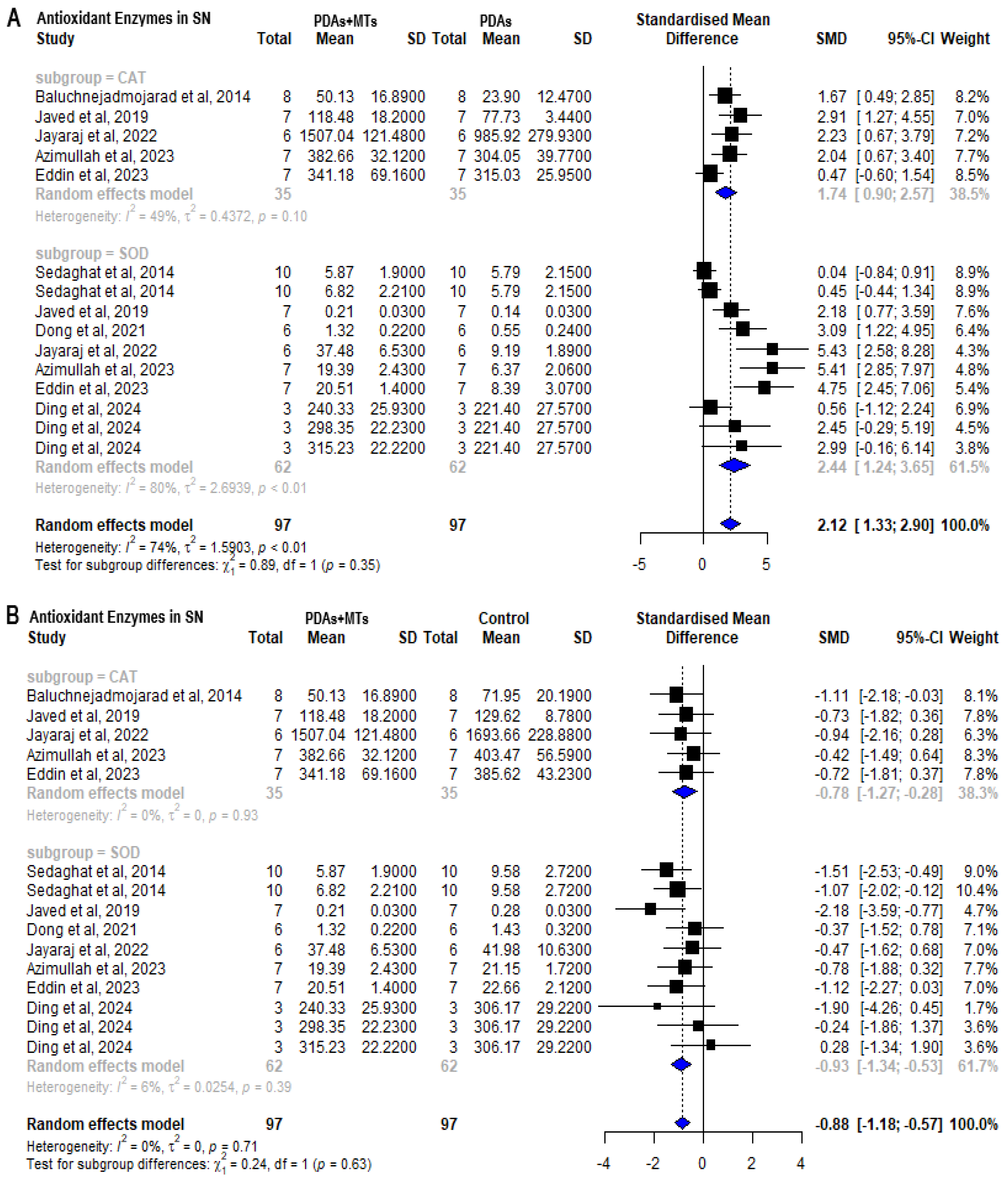
2.6. Antioxidant Properties of MTs
2.7. Anti-Inflammatory Properties
2.8. Limitations Section
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Data Extraction and Evaluation of Risk of Bias
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-3-527-31786-8/978-3-527-60994-9. [Google Scholar]
- Milawati, H.; Harneti, D.; Maharani, R.; Nurlelasari, N.; Hidayat, A.T.; Azmi, M.N.; Shiono, Y.; Supratman, U. Caryophyllene-Type Sesquiterpenoids from the Stembark of Aglalia Harmsiana and Their Cytotoxic Activity against MCF-7 Breast Cancer Cells. Molekul 2019, 14, 126–132. [Google Scholar]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant Terpenes: Defense Responses, Phylogenetic Analysis, Regulation and Clinical Applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Riyadi, S.A.; Naini, A.A.; Supratman, U. Sesquiterpenoids from Meliaceae Family and Their Biological Activities. Molecules 2023, 28, 4874. [Google Scholar] [CrossRef] [PubMed]
- Stephane, F.F.Y.; Jules, B.K.J. Terpenoids as Important Bioactive Constituents of Essential Oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; InTech Open: London, UK, 2020; pp. 1–15. [Google Scholar]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini-Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef]
- Baginska, S.; Golonko, A.; Swislocka, R.; Lewandowski, W. Monoterpenes as medicinal agents: Exploring the pharmaceutical potential of p-cymene, p-cymenene, and γ-terpinene. Acta Pol. Pharm.—Drug Res. 2023, 80, 879–892. [Google Scholar] [CrossRef]
- Graßmann, J. Terpenoids as Plant Antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar]
- De Cássia Da Silveira E Sá, R.; Andrade, L.; De Sousa, D. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Alves-Silva, J.; Salgueiro, L. Plant Monoterpenes and Essential Oils as Potential Anti-Ageing Agents: Insights from Preclinical Data. Biomedicines 2024, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic Control of the Dopamine Transporter in Neurotransmission and Homeostasis. NPJ Park. Dis. 2021, 7, 22. [Google Scholar] [CrossRef]
- Formisano, R.; Rosikon, K.D.; Singh, A.; Dhillon, H.S. The Dopamine Membrane Transporter Plays an Active Modulatory Role in Synaptic Dopamine Homeostasis. J. Neurosci. Res. 2022, 100, 1551–1559. [Google Scholar] [CrossRef]
- Cramb, K.M.L.; Beccano-Kelly, D.; Cragg, S.J.; Wade-Martins, R. Impaired Dopamine Release in Parkinson’s Disease. Brain 2023, 146, 3117–3132. [Google Scholar] [CrossRef]
- Kägi, G.; Bhatia, K.P.; Tolosa, E. The Role of DAT-SPECT in Movement Disorders. J. Neurol. Neurosurg. Psychiatry 2010, 81, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Büeler, H. Impaired Mitochondrial Dynamics and Function in the Pathogenesis of Parkinson’s Disease. Exp. Neurol. 2009, 218, 235–246. [Google Scholar] [CrossRef]
- Zuo, L.; Motherwell, M.S. The Impact of Reactive Oxygen Species and Genetic Mitochondrial Mutations in Parkinson’s Disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef]
- Hattori, N.; Mizuno, Y. Mitochondrial Dysfunction in Parkinson’s Disease. Nippon. Rinsho Jpn. J. Clin. Med. 2002, 60 (Suppl. S4), 406–411. [Google Scholar] [CrossRef]
- De Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira Júnior, R.G.; Gama e Silva, M.; De Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araújo, M.T.; Coutinho, H.D.M. Essential Oils and Their Major Compounds in the Treatment of Chronic Inflammation: A Review of Antioxidant Potential in Preclinical Studies and Molecular Mechanisms. Oxidative Med. Cell. Longev. 2018, 2018, 6468593. [Google Scholar]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Ansari, M.A.; Ahmad, M.; Saleem, S.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotection by Crocetin in a Hemi-Parkinsonian Rat Model. Pharmacol. Biochem. Behav. 2005, 81, 805–813. [Google Scholar] [PubMed]
- Fasano, M. Biochemistry of Parkinson’s Disease—Insights from Cellular Models, Animal Models and Human Tissue Specimens Obtained by Autopsy. FEBS J. 2012, 279, 1145. [Google Scholar]
- Ding, L.; Wang, L.; Yang, J.; Jiang, C.; Sun, X.; Huang, H.; Zhan, X.; Liu, F.; Zhang, Q. (+)-Borneol Protects Dopaminergic Neuronal Loss in Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine-Induced Parkinson’s Disease Mice: A Study of Dopamine Level Using In Vivo Brain Microdialysis. ACS Chem. Neurosci. 2024, 15, 2308–2321. [Google Scholar]
- Akan, T.; Aydın, Y.; Korkmaz, O.T.; Ulupınar, E.; Saydam, F. The Effects of Carvacrol on Transient Receptor Potential (TRP) Channels in an Animal Model of Parkinson’s Disease. Neurotox. Res. 2023, 41, 660–669. [Google Scholar]
- Baluchnejadmojarad, T.; Hassanshahi, J.; Roghani, M.; Mansouri, M.; Raoufi, S. Protective Effect of Carvacrol in 6-Hydroxydopamine Hemi-Parkinsonian Rat Model. J. Basic Clin. Pathophysiol. 2014, 2, 29–34. [Google Scholar]
- Dati, L.M.; Ulrich, H.; Real, C.C.; Feng, Z.P.; Sun, H.S.; Britto, L.R. Carvacrol Promotes Neuroprotection in the Mouse Hemiparkinsonian Model. Neuroscience 2017, 356, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, H.; Rajaei, Z.; Alaei, H.; Shahidani, S. Chronic Treatment with Carvacrol Improves Passive Avoidance Memory in a Rat Model of Parkinson’s Disease. Arq. Neuro-Psiquiatr. 2018, 76, 71–77. [Google Scholar]
- Hamzehloei, L.; Rezvani, M.E.; Rajaei, Z. Effects of Carvacrol and Physical Exercise on Motor and Memory Impairments Associated with Parkinson’s Disease. Arq. Neuro-Psiquiatr. 2019, 77, 493–500. [Google Scholar]
- Lins, L.C.R.F.; Souza, M.F.; Bispo, J.M.M.; Gois, A.M.; Melo, T.C.S.; Andrade, R.A.S.; Quintans-Junior, L.J.; Ribeiro, A.M.; Silva, R.H.; Santos, J.R. Carvacrol Prevents Impairments in Motor and Neurochemical Parameters in a Model of Progressive Parkinsonism Induced by Reserpine. Brain Res. Bull. 2018, 139, 9–15. [Google Scholar]
- Manouchehrabadi, M.; Farhadi, M.; Azizi, Z.; Torkaman-Boutorabi, A. Carvacrol Protects Against 6-Hydroxydopamine-Induced Neurotoxicity in In Vivo and In Vitro Models of Parkinson’s Disease. Neurotox. Res. 2020, 37, 156–170. [Google Scholar] [CrossRef]
- Ribeiro, C.T.; Gasparotto, J.; Petiz, L.L.; Brum, P.O.; Peixoto, D.O.; Kunzler, A.; da Rosa Silva, H.T.; Bortolin, R.C.; Almeida, R.F.; Quintans-Junior, L.J. Oral Administration of Carvacrol/β-Cyclodextrin Complex Protects against 6-Hydroxydopamine-Induced Dopaminergic Denervation. Neurochem. Int. 2019, 126, 27–35. [Google Scholar]
- Shah, S.; Tryphena, K.P.; Singh, G.; Kulkarni, A.; Pinjala, P.; Khatri, D.K. Neuroprotective Role of Carvacrol via Nrf2/HO-1/NLRP3 Axis in Rotenone-Induced PD Mice Model. Brain Res. 2024, 1836, 148954. [Google Scholar] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Parekh, K.A.; Ojha, S.K.; Beiram, R. Effect of Citronellol on Oxidative Stress, Neuroinflammation and Autophagy Pathways in an in Vivo Model of Parkinson’s Disease. Heliyon 2022, 8, e11434. [Google Scholar]
- Rekha, K.R.; Selvakumar, G.P.; Sethupathy, S.; Santha, K.; Sivakamasundari, R.I. Geraniol Ameliorates the Motor Behavior and Neurotrophic Factors Inadequacy in MPTP-Induced Mice Model of Parkinson’s Disease. J. Mol. Neurosci. 2013, 51, 851–862. [Google Scholar]
- Rekha, K.R.; Selvakumar, G.P.; Santha, K.; Sivakamasundari, R.I. Geraniol Attenuates α-Synuclein Expression and Neuromuscular Impairment through Increase Dopamine Content in MPTP Intoxicated Mice by Dose Dependent Manner. Biochem. Biophys. Res. Commun. 2013, 440, 664–670. [Google Scholar] [PubMed]
- Rekha, K.R.; Selvakumar, G.P. Gene Expression Regulation of Bcl2, Bax and Cytochrome-C by Geraniol on Chronic MPTP/Probenecid Induced C57BL/6 Mice Model of Parkinson’s Disease. Chem.-Biol. Interact. 2014, 217, 57–66. [Google Scholar]
- Eddin, L.B.; Azimullah, S.; Jha, N.K.; Nagoor Meeran, M.F.; Beiram, R.; Ojha, S. Limonene, a Monoterpene, Mitigates Rotenone-Induced Dopaminergic Neurodegeneration by Modulating Neuroinflammation, Hippo Signaling and Apoptosis in Rats. Int. J. Mol. Sci. 2023, 24, 5222. [Google Scholar] [CrossRef]
- de Lucena, J.D.; Gadelha-Filho, C.V.J.; da Costa, R.O.; de Araújo, D.P.; Lima, F.A.V.; Neves, K.R.T.; de Barros Viana, G.S. L-Linalool Exerts a Neuroprotective Action on Hemiparkinsonian Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1077–1088. [Google Scholar]
- Chang, W.-H.; Hsu, H.-T.; Lin, C.-C.; An, L.-M.; Lee, C.-H.; Ko, H.-H.; Lin, C.-L.; Lo, Y.-C. Linalool, a Fragrance Compound in Plants, Protects Dopaminergic Neurons and Improves Motor Function and Skeletal Muscle Strength in Experimental Models of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 2514. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, D.; Zhang, X.; Zhou, A.; Su, Y.; He, D.; Fu, S.; Gao, F. Menthol Protects Dopaminergic Neurons against Inflammation-Mediated Damage in Lipopolysaccharide (LPS)-Evoked Model of Parkinson’s Disease. Int. Immunopharmacol. 2020, 85, 106679. [Google Scholar] [CrossRef] [PubMed]
- Azimullah, S.; Jayaraj, R.L.; Meeran, M.F.; Jalal, F.Y.; Adem, A.; Ojha, S.; Beiram, R. Myrcene Salvages Rotenone-Induced Loss of Dopaminergic Neurons by Inhibiting Oxidative Stress, Inflammation, Apoptosis, and Autophagy. Molecules 2023, 28, 685. [Google Scholar] [CrossRef]
- Tancheva, L.P.; Lazarova, M.I.; Alexandrova, A.V.; Dragomanova, S.T.; Nicoletti, F.; Tzvetanova, E.R.; Hodzhev, Y.K.; Kalfin, R.E.; Miteva, S.A.; Mazzon, E. Neuroprotective Mechanisms of Three Natural Antioxidants on a Rat Model of Parkinson’s Disease: A Comparative Study. Antioxidants 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Silva-Martins, S.; Beserra-Filho, J.I.A.; Maria-Macêdo, A.; Custódio-Silva, A.C.; Soares-Silva, B.; Silva, S.P.; Lambertucci, R.H.; Silva, R.H.; Dos Santos, J.R.; Gandhi, S.R. Myrtenol Complexed with Β-cyclodextrin Ameliorates Behavioural Deficits and Reduces Oxidative Stress in the Reserpine-induced Animal Model of Parkinsonism. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1488–1499. [Google Scholar]
- Anis, E.; Zafeer, M.F.; Firdaus, F.; Islam, S.N.; Khan, A.A.; Hossain, M.M. Perillyl Alcohol Mitigates Behavioural Changes and Limits Cell Death and Mitochondrial Changes in Unilateral 6-OHDA Lesion Model of Parkinson’s Disease through Alleviation of Oxidative Stress. Neurotox. Res. 2020, 38, 461–477. [Google Scholar] [CrossRef]
- Srivastava, R.; Choudhury, P.K.; Dev, S.K.; Rathore, V. Neuroprotective Effect of α-Pinene Self-Emulsifying Nanoformulation against 6-OHDA Induced Neurotoxicity on Human SH-SY5Y Cells and Its in Vivo Validation for Anti-Parkinson’s Effect. J. Biochem. Mol. Toxicol. 2021, 35, e22902. [Google Scholar] [CrossRef]
- Srivastava, R.; Choudhury, P.K.; Dev, S.K.; Rathore, V. Alpha-Pine Self-Emulsifying Nano Formulation Attenuates Rotenone and Trichloroethylene-Induced Dopaminergic Loss. Int. J. Neurosci. 2024, 24, 1–18. [Google Scholar] [CrossRef]
- Yang, W.; Wei, Y.; Sun, J.; Yao, C.; Ai, F.; Ding, H. Safranal Exerts a Neuroprotective Effect on Parkinson’s Disease with Suppression of NLRP3 Inflammation Activation. Mol. Biol. Rep. 2024, 51, 593. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Meeran, M.N.; Ansari, S.A.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef]
- Nourmohammadi, S.; Yousefi, S.; Manouchehrabadi, M.; Farhadi, M.; Azizi, Z.; Torkaman-Boutorabi, A. Thymol Protects against 6-Hydroxydopamine-Induced Neurotoxicity in in Vivo and in Vitro Model of Parkinson’s Disease via Inhibiting Oxidative Stress. BMC Complement. Med. Ther. 2022, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Hamdan, A.M.E.; Mohammed, A.A.; Atwa, A.M.; Kozman, M.R.; Ibrahim, A.M.; Motawea, S.M.; Selim, H.M.R.M.; Tohamy, S.T.K.; El-Din, M.N. Neuroprotective Effects of Some Nutraceuticals against Manganese-Induced Parkinson’s Disease in Rats: Possible Modulatory Effects on TLR4/NLRP3/NF-ΚB, GSK-3β, Nrf2/HO-1, and Apoptotic Pathways. Pharmaceuticals 2022, 15, 1554. [Google Scholar] [PubMed]
- Sedaghat, R.; Roghani, M.; Khalili, M. Neuroprotective Effect of Thymoquinone, the Nigella Sativa Bioactive Compound, in 6-Hydroxydopamine-Induced Hemi-Parkinsonian Rat Model. Iran. J. Pharm. Res. IJPR 2014, 13, 227. [Google Scholar]
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone Exerts Neuroprotective Effect in Animal Model of Parkinson’s Disease. Toxicol. Lett. 2017, 276, 108–114. [Google Scholar]
- Ardah, M.T.; Merghani, M.M.; Haque, M.E. Thymoquinone Prevents Neurodegeneration Against MPTP In Vivo and Modulates α-Synuclein Aggregation in Vitro. Neurochem. Int. 2019, 128, 115–126. [Google Scholar]
- Dong, J.; Zhang, X.; Wang, S.; Xu, C.; Gao, M.; Liu, S.; Li, X.; Cheng, N.; Han, Y.; Wang, X. Thymoquinone Prevents Dopaminergic Neurodegeneration by Attenuating Oxidative Stress via the Nrf2/ARE Pathway. Front. Pharmacol. 2021, 11, 615598. [Google Scholar]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A Rotarod Test for Evaluation of Motor Skill Learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef]
- Sanberg, P.R.; Bunsey, M.D.; Giordano, M.; Norman, A.B. The Catalepsy Test: Its Ups and Downs. Behav. Neurosci. 1988, 102, 748–759. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and Thiobarbituric Acid-Reactivity as Diagnostic Indices of Lipid Peroxidation and Peroxidative Tissue Injury. Free. Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Stoker, T.B.; Barker, R.A. Recent Developments in the Treatment of Parkinson’s Disease. F1000Research 2020, 9, 862. [Google Scholar] [CrossRef]
- Diederich, M. Natural Products Target the Hallmarks of Chronic Diseases. Biochem. Pharmacol. 2020, 173, 113828. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Gegechkori, V.; Morton, D.W. Anxiolytic Terpenoids and Aromatherapy for Anxiety and Depression. In Reviews on New Drug Targets in Age-Related Disorders; Springer: Berlin/Heidelberg, Germany, 2020; pp. 283–296. [Google Scholar]
- Mony, T.J.; Elahi, F.; Choi, J.W.; Park, S.J. Neuropharmacological Effects of Terpenoids on Preclinical Animal Models of Psychiatric Disorders: A Review. Antioxidants 2022, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Ewais, O.; Abdel-Tawab, H.; El-Fayoumi, H.; Aboelhadid, S.M.; Al-Quraishy, S.; Falkowski, P.; Abdel-Baki, A.-A.S. Antioxidant Properties of D-Limonene and Its Nanoemulsion Form Enhance Its Anticoccidial Efficiency in Experimentally Infected Broilers with Eimeria Tenella: An in Vitro and in Vivo Study. Vet. Res. Commun. 2024, 48, 3711–3725. [Google Scholar] [CrossRef] [PubMed]
- Karimi Afshar, S.; Rostamzadeh, F.; Bigdeli, M.R.; Mortazavi Moghadam, F. Myrtenol-Loaded Fatty Acid Nanocarriers Protect Rat Brains Against Ischemia–Reperfusion Injury: Antioxidant and Anti-Inflammatory Effects. Chem. Biol. Drug Des. 2024, 104, e14633. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.; Gammoh, O.; Bseiso, Y.; Wedyan, M. Protective Effects of α-Pinene against Carbon Tetrachloride-Induced Cardiac Injury in Wistar Rats: Modulation of Antioxidant and Inflammatory Responses. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 2845–2854. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, C.; Yu, J. Natural Monoterpenes as Potential Therapeutic Agents against Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 2429. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; Galle, M.; Crespo, R. 1, 8-Cineole Promotes G0/G1 Cell Cycle Arrest and Oxidative Stress-Induced Senescence in HepG2 Cells and Sensitizes Cells to Anti-Senescence Drugs. Life Sci. 2020, 243, 117271. [Google Scholar]
- Cavalcanti, B.C.; Magalhães, I.L.; Rocha, D.D.; Stefânio Barreto, F.; de Andrade Neto, J.B.; Magalhães, H.I.F.; dos Santos, C.C.; de Moraes, M.O. In Vitro Evaluation of Cytotoxic Potential of Essential Oil Extracted from Leaves of Croton Heliotropiifolius Kunth in Human Tumor Cells. J. Toxicol. Environ. Health Part A 2024, 87, 91–107. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool Attenuates Oxidative Stress and Mitochondrial Dysfunction Mediated by Glutamate and NMDA Toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef]
- Prasad, S.N. Muralidhara Mitigation of Acrylamide-Induced Behavioral Deficits, Oxidative Impairments and Neurotoxicity by Oral Supplements of Geraniol (a Monoterpene) in a Rat Model. Chem.-Biol. Interact. 2014, 223, 27–37. [Google Scholar] [CrossRef]
- Crespo, R.; Rodenak-Kladniew, B.E.; Castro, M.A.; Soberón, M.V.; Lavarías, S.M.L. Induction of Oxidative Stress as a Possible Mechanism by Which Geraniol Affects the Proliferation of Human A549 and HepG2 Tumor Cells. Chem.-Biol. Interact. 2020, 320, 109029. [Google Scholar] [CrossRef]
- Santos, M.R.V.; Moreira, F.V.; Fraga, B.P.; De Souza, D.P.; Bonjardim, L.R.; Quintans-Junior, L.J. Cardiovascular Effects of Monoterpenes: A Review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef]
- Sousa, L.D.R.; Viana, N.R.; Coêlho, A.G.; Barbosa, C.D.O.; Barros, D.S.L.; Martins, M.D.C.D.C.E.; Ramos, R.M.; Arcanjo, D.D.R. Use of Monoterpenes as Potential Therapeutics in Diabetes Mellitus: A Prospective Review. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1512974. [Google Scholar] [CrossRef] [PubMed]
- Barras, B.J.; Ling, T.; Rivas, F. Recent Advances in Chemistry and Antioxidant/Anticancer Biology of Monoterpene and Meroterpenoid Natural Product. Molecules 2024, 29, 279. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Kasprzak-Drozd, K.; Oniszczuk, A.; Borowicz-Reutt, K. Activity of Selected Group of Monoterpenes in Alzheimer’s Disease Symptoms in Experimental Model Studies—A Non-Systematic Review. Int. J. Mol. Sci. 2021, 22, 7366. [Google Scholar] [CrossRef]
- Silva, E.A.P.; Santos, D.M.; de Carvalho, F.O.; Menezes, I.A.C.; Barreto, A.S.; Souza, D.S.; Quintans-Junior, L.J.; Santos, M.R. V Monoterpenes and Their Derivatives as Agents for Cardiovascular Disease Management: A Systematic Review and Meta-Analysis. Phytomedicine 2021, 88, 153451. [Google Scholar]
- Quintans, J.S.S.; Shanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.G.d.S.; Picot, L.; Quintans-Júnior, L.J. Monoterpenes Modulating Cytokines—A Review. Food Chem. Toxicol. 2019, 123, 233–257. [Google Scholar] [CrossRef]
- Ainseba, N.; Soulimane, A.; Mami, I.R.; Dib, M.E.A.; Muselli, A. Evaluation of the Antioxidant and Anti-Inflammatory Activity of the Anacyclus valentinus L. Essential Oil and Its Oxygenated Fraction. Comb. Chem. High Throughput Screen. 2024, 27, 765–772. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Suarez y Bruno. MetaStat: Software Estadístico Para Metaanálisis. Estadística y Biometría. FCA.UNC. CONICET. Argentina. Patente de Invención. Registro N°5380320. 2024. Available online: https://Github.Com/FrancoMSuarez/MetaStats (accessed on 15 August 2024).
- Borenstein, M. Research Note: In a Meta-Analysis, the I2 Index Does Not Tell Us How Much the Effect Size Varies across Studies. J. Physiother. 2020, 66, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jávega-Cometto, M.; Naranjo-Viteri, A.J.; Champarini, L.G.; Hereñú, C.B.; Crespo, R. Plant-Derived Monoterpene Therapies in Parkinson’s Disease Models: Systematic Review and Meta-Analysis. Plants 2025, 14, 999. https://doi.org/10.3390/plants14070999
Jávega-Cometto M, Naranjo-Viteri AJ, Champarini LG, Hereñú CB, Crespo R. Plant-Derived Monoterpene Therapies in Parkinson’s Disease Models: Systematic Review and Meta-Analysis. Plants. 2025; 14(7):999. https://doi.org/10.3390/plants14070999
Chicago/Turabian StyleJávega-Cometto, Matías, Aracely J. Naranjo-Viteri, Leandro G. Champarini, Claudia B. Hereñú, and Rosana Crespo. 2025. "Plant-Derived Monoterpene Therapies in Parkinson’s Disease Models: Systematic Review and Meta-Analysis" Plants 14, no. 7: 999. https://doi.org/10.3390/plants14070999
APA StyleJávega-Cometto, M., Naranjo-Viteri, A. J., Champarini, L. G., Hereñú, C. B., & Crespo, R. (2025). Plant-Derived Monoterpene Therapies in Parkinson’s Disease Models: Systematic Review and Meta-Analysis. Plants, 14(7), 999. https://doi.org/10.3390/plants14070999








