Differences in Seed Weight, Amino Acid, Fatty Acid, Oil, and Squalene Content in γ-Irradiation-Developed and Commercial Amaranth Varieties (Amaranthus spp.)
Abstract
:1. Introduction
2. Results and Discussion
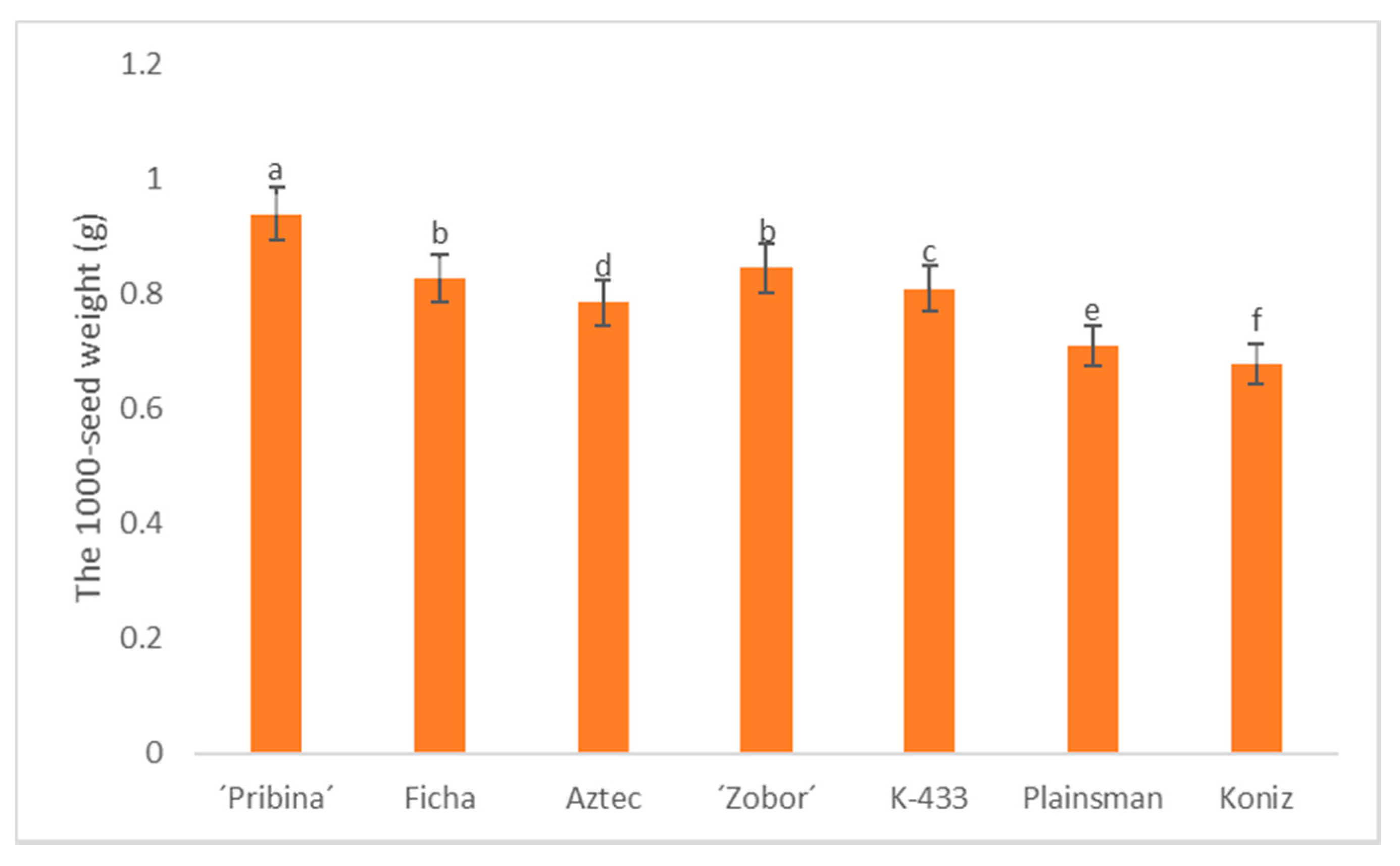
2.1. Evaluation of 1000-seed Weight
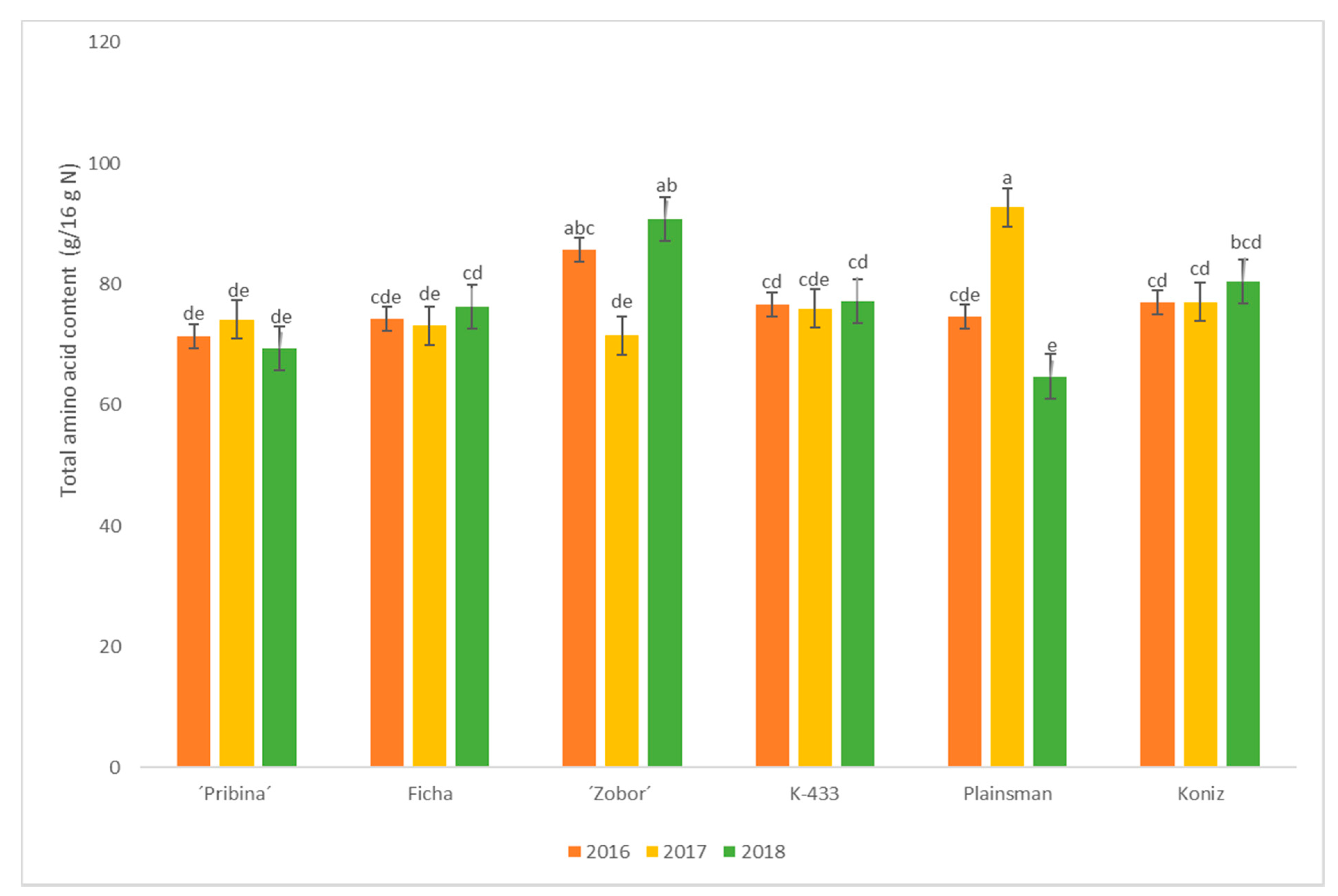
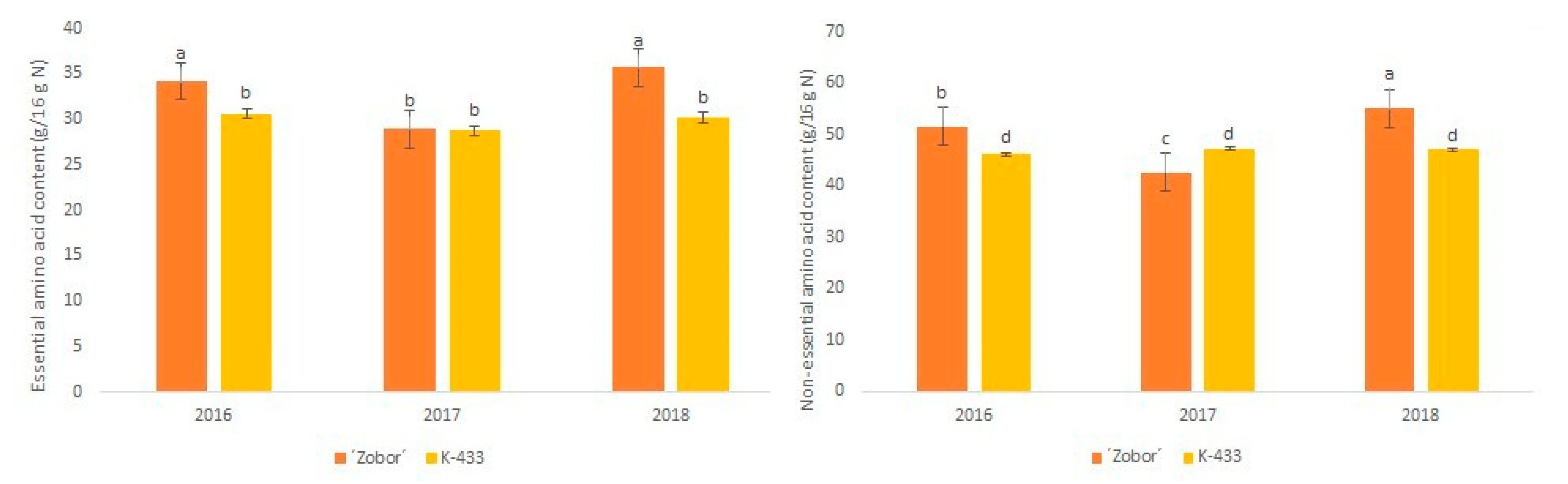
2.2. Amino Acid Analysis
2.3. Total Oil and Squalene Content
2.4. Fatty Acid Composition
3. Materials and Methods
3.1. Plant Material and 1000-seed Weight
3.2. Amino Acid Analysis
3.3. Oil Analysis
3.3.1. Oil Content and Squalene Content
3.3.2. Fatty Acid Composition
3.4. Experiment Design and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grobelnik, S.M.; Turinek, M.; Jakop, M.; Bavec, M.; Bavec, F. Grain Amaranth as an alternative and perspective crop in temperate climate. J. Geogr. 2010, 5, 135–145. [Google Scholar]
- Akin-Idowu, P.E.; Odunola, O.A.; Gbadegesin, M.A.; Oke, A.; Orkpeh, U. Assessment of the protein quality of twenty nine grain amaranth (Amaranthus spp. L.) accessions using amino acid analysis and one-dimensional electrophoresis. Afr. J. Biotechnol. 2013, 12, 1802–1810. [Google Scholar] [CrossRef] [Green Version]
- Gorinstein, S.; Pawelzik, E.; Delgado-Licon, E.; Haruenkit, R.; Weisz, M.; Trakhtenberg, S. Characterisation of pseudocereal proteins by protein and amino acid analyses. J. Sci. Food Agric. 2002, 82, 886–891. [Google Scholar] [CrossRef]
- Mota, C.; Santos, M.; Mauro, R.; Samman, N.; Matos, A.S.; Torres, D.; Castanheira, I. Protein Content Amino Acids Profile Pseudocereals. Food Chem. 2016, 193, 55–61. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional components of amaranth seeds and vegetables: A review on composition, properties and uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Sujak, A.; Dziwulska-Hunek, A.; Kornarzyński, K. Compositional and nutritional values of amaranth seeds after pre-sowing He-Ne laser light and alternating magnetic field treatment. Int. Agrophys. 2009, 23, 81–86. [Google Scholar]
- Bressani, R.; Elias, L.G.; Garcia-Soto, A. Limiting amino acids in raw and processed amaranth grain from biological tests. Plant. Foods Hum. Nutr. 1989, 39, 223–234. [Google Scholar] [CrossRef]
- Pedersen, B.; Kalinowski, L.S.; Eggum, B.O. The nutritive value of amaranth grain (Amaranthus caudatus). Plant. Foods Hum. Nutr. 1987, 36, 309–324. [Google Scholar] [CrossRef]
- Bejosano, F.P.; Corke, H. Protein quality evaluation of Amaranthus wholemeal flours and protein concentrates. J. Sci. Food Agric. 1998, 76, 100–106. [Google Scholar] [CrossRef]
- Grobelnik, S.M.; Turinek, M.; Jakop, M.; Bavec, M.; Bavec, F. Nutrition value and use of grain amaranth: Potential future application in bread making. Agricultura 2009, 6, 43–53. [Google Scholar]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 2009, 60, 240–257. [Google Scholar] [CrossRef] [PubMed]
- He, H.P.; Cai, Y.; Sun, M.; Corke, H. Extraction and purification of squalene from amaranthus grain. J. Agric. Food Chem. 2002, 50, 368–372. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Kang, Y.-J.; Che, L. Composition and thermal characteristics of seed oil obtained from Chinese Amaranth. LWT Food Sci. Technol. 2019, 111, 39–45. [Google Scholar] [CrossRef]
- Martirosyan, D.M.; Miroshnichenko, L.A.; Kulakova, S.N.; Pogojeva, A.V.; Zoloedov, V.I. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmalogical activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef] [PubMed]
- Gunes, F.E. Medical use of squalene as a natural antioxidant. J. Marmara Univ. Inst. Health Sci. 2013, 33, 220–228. [Google Scholar]
- Smith, T.J. Squalene: Potential chemopreventive agent. Expert Opin. Investig. Drugs 2000, 9, 1841–1848. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant sources, extraction methods, and use of squalene. Int. J. Agron. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Caselato-Sousa, V.M.; Amaya-Farfán, J. State of knowledge of amaranth grain: A comprehensive review. J. Food Sci. 2012, 77, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From zero to hero: The past, present and future of grain amaranth breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Lyu, J.I.; Lee, M.K.; Kim, J.M.; Hung, N.N.; Hong, M.J.; Kim, J.B.; Bae, C.H.; Kwon, S.-J. Construction of soybean mutant diversity pool (MDP) lines and an analysis of their genetic relationships and associations using TRAP markers. Agronomy 2020, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Gajdošová, A.; Libiaková, G.; Fejer, J. Improvement of selected Amaranthus cultivars by means of mutation induction and biotechnological approaches. In Breeding of Neglected and Under-Utilized Crops. Spices and Herbs; Science Publisher: Enfield, NH, USA, 2007; pp. 151–169. [Google Scholar]
- Gajdošová, A.; Hricová, A.; Libiaková, G.; Fejér, J. New variety of amaranth ’Zobor’ bred by mutagenesis from interspecific hybrid Amaranthus hypochondriacus L. × Amaranthus hybridus L. 2018. Available online: https://www.researchgate.net/publication/324007593_New_variety_of_amaranth_%27ZOBOR%27_bred_by_mutagenesis_from_the_interspecific_hybrid_Amaranthus_hypochondriacus_L_x_Amaranthus_hybridus_L (accessed on 17 September 2020).
- Hricová, A.; Kečkešová, M.; Gálová, Z.; Libiaková, G.; Gajdošová, A. Investigation of protein profile changes in amaranth seeds after radiation mutagenesis. Chem. Listy 2011, 105, 542–545. [Google Scholar]
- Hricová, A.; Žiarovská, J.; Suhaj, M.; Lancíková, V. Significantly lower content of antinutritional soluble oxalate in amaranth mutant lines developed by radiation mutagenesis. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 820–823. [Google Scholar]
- Kečkešová, M.; Gálová, Z.; Hricová, A. Changes of protein profiles in amaranth mutant lines. J. Microbiol. Biotechnol. Food Sci. 2012, 1, 1129–1135. [Google Scholar]
- Kečkešová, M.; Palenčárová, E.; Gálová, Z.; Gažo, J.; Hricová, A. Nutritional quality of grain amaranths (Amaranthus L.) compared to putative mutant lines. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 1716–1724. [Google Scholar]
- Vujacic, V.; Momirovic, G.S.; Perovic, D.; Nikolic, A. Variability, heritability and classification of Amaranthus, L. genotypes by chierarchial analysis. Rom. Agric. Res. 2014, 31, 59–67. [Google Scholar]
- Gulmezoglu, N.; Aytac, Z. Response of grain and protein yields of triticale varieties at different levels of applied nitrogen fertilizer. Afr. J. Agric. Res. 2012, 5, 2563–2569. [Google Scholar]
- Moshatati, A.; Gharineh, M.H. Effect of grain weight on germination and seed vigor of wheat. Int. J. Agric. Crop. Sci. 2012, 4, 458–460. [Google Scholar]
- Kaul, H.P.; Aufhammer, W.; Laible, B.; Nalborczyk, E.; Pirog, S.; Wasiak, K. The suitability of amaranth genotypes for grain and fodder use in Central Europe. Die Bodenkult. 1996, 43, 173–181. [Google Scholar]
- Feckova, J.; Habán, M.; Habánová, M. Relations between some production traits of selected genotypes of amaranth (Amaranthus L.). Acta Fytotech. Et Zootech. 2003, 6, 1–5. [Google Scholar]
- Gimplinger, D.M.; Dobos, G.; Schönlechner, R.; Kaul, H.-P. Yield and quality of grain amaranth (Amaranthus sp.) in Eastern Austria. Plant. Soil Environ. 2007, 53, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Janovská, D.; Čepková, P.H.; Džunková, M. Characterization of the amaranth genetic resources in the Czech gene bank. In Genetic Diversity in Plants; Caliskan, M., Ed.; IntechOpen: London, UK, 2012; pp. 457–478. [Google Scholar]
- Rivelli, A.R.; Gherbin, P.; De Maria, S.; Pizza, S. Field evaluation of Amaranthus species for seed and biomass yields in southern Italy. Ital. J. Agron. 2008, 3, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Hricová, A.; Fejér, J.; Libiaková, G.; Szabóová, M.; Gažo, J.; Gajdošová, A. Characterization of phenotypic and nutritional properties of valuable Amaranthus cruentus L. mutants. Turk. J. Agric. For. 2016, 40, 761–771. [Google Scholar] [CrossRef]
- Dodok, L.; Modhir, A.; Buchtová, V.; Halásová, G.; Poláček, I. Importance and utilization of amaranth in food industry. Part 2. Composition of amino acids and fatty acids. Nahrung 1997, 41, 108–110. [Google Scholar] [CrossRef]
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Gohara, A.K.; Souza, A.H.P.; De Souza, N.E.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Evaluation of nutritional compound in new amaranth and quinoa cultivars. Food Sci. Technol. 2013, 33, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Tömösközi, S.; Baracskai, I.; Schönlechner, R.; Berghofer, E.; Läsztity, R. Comparative study of composition and technological quality of amaranth: I. Gross chemical composition, amino acid and mineral content. Acta Aliment. 2009, 38, 341–347. [Google Scholar] [CrossRef]
- Nimbalkar, M.S.; Pai, S.P.; Pawar, N.V.; Oulkar, D.; Dixit, G.B. Free amino acid profiling in grain Amaranth using LC-MS/MS. Food Chem. 2012, 134, 2565–2569. [Google Scholar] [CrossRef]
- Shukla, A.; Srivasta, N.; Suneja, P.; Yadav, S.K.; Hussain, Z.; Rana, J.C.; Yadav, S. Untapped amaranth (Amaranthus spp.) genetic diversity with potential for nutritional enhancement. Genet. Resour. Crop. Evol. 2017, 65, 243–253. [Google Scholar] [CrossRef]
- Pisaříková, B.; Zralý, Z.; Kráčmar, S.; Trčková, M.; Herzig, I. Nutritional value of amaranth (genus Amaranthus, L.) grain in diets for broiler chikens. Czech. J. Anim. Sci. 2005, 50, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Prugar, J. Kvalita Rostlinných Produktů na Prahu 3. Tisíciletí; Výzkumný ústav Pivovarský a Sladařský ve Spolupráci s Komisí Jakosti Rostlinných Produktů ČAZV: Praha, Česká Republika, 2008; p. 327. ISBN 9788086576282. [Google Scholar]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.M.; Hellström, J.; Pihlanto, A. Nutritional value of commercial protein-rich plant products. Plant. Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugbe, J.X.; Salifu, B.; Opoku, N. Potential of gamma rays to improve grain yield and nutritional quality of pearl millet (Pennisetum glaucum L.): A rewiew. Elixir Int. J. 2015, 79, 30667–30671. [Google Scholar]
- Mehlo, L.; Mbambo, Z.; Bado, S.; Lin, J.; Moagi, S.M.; Buthelezi, S.; Stoychev, S.; Chikwamba, R. Induced protein polymorphisms and nutritional quality of gamma irradiation mutants of sorghum. Mutat. Res. 2013, 279, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Islam, T.; Rabbani, G.; Oba, S. Genetic variation and interrelationships among antioxidant, quality, and agronomic traits in vegetable amaranth. Turk. J. Agric. For. 2016, 40, 526–535. [Google Scholar] [CrossRef]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Srivastava, J.; Singh, N.; Singh, S.P. Mineral profile and variability in vegetable amaranth (Amaranthus tricolor). Plant. Foods Hum. Nutr. 2006, 61, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Pandey, A.C.H.; Kumar, A.R.A. Genetic interrelationship among nutritional and quantitative traits in the vegetable amaranth. Crop. Breed. Appl. Biotechnol. 2010, 10, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Bressani, R.; Sánchez-Marroquín, A.; Morales, E. Chemical composition of grain amaranth cultivars and effects of processing on their nutritional quality. Food Rev. Int. 1992, 8, 23–49. [Google Scholar] [CrossRef]
- Mlakar, S.G.; Jakop, M.; Turinek, M.; Robačer, M.; Bavec, M.; Bavec, F. Protein concentration and amino acid composition in grain amaranth (Amaranthus cruentus L.) as affected by sowing date and nitrogen fertilization. Afr. J. Agric. Res. 2012, 7, 5238–5246. [Google Scholar]
- Hlinková, A.; Bednárová, A.; Havrlentová, M.; Šupová, J.; Čičová, I. Evaluation of fatty acid composition among selected amaranth grains grown in two consecutive years. Biologia 2013, 68, 641–650. [Google Scholar] [CrossRef]
- He, H.P.; Corke, H. Oil and squalene in Amaranthus grain and leaf. J. Agric. Food Chem. 2003, 51, 7913–7920. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, S.B.; Berdiev, N.S.; Ishimov, U.J.; Olimjonov, S.S.; Ziyavitdinov, J.F.; Asrprpv, A.M.; Salikhov, S.I. Chemical composition and biological activity of seed oil of amaranth varieties. Nova Biotechnol. Et Chim. 2018, 17, 66–73. [Google Scholar] [CrossRef]
- Ghimire, G.P.; Thuan, N.H.; Koirala, N.; Sohng, J.K. Advances in biochemistry and microbial production of squalene and its derivates. J. Microbiol. Biotechnol. 2016, 26, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Kraujalis, P.K.; Venskutonis, P.R. Supercritical carbon dioxide extraction of squalene and tocopherols from amaranth and assessment of extracts antioxidant activity. J. Supercrit. Fluids 2013, 80, 78–85. [Google Scholar] [CrossRef]
- Spanova, M.; Daum, G. Squalene- biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Khamar, R.P.; Jasrai, Y.T. Nutraceutical profile of selected oils, distillates and butters. Asian J. Exp. Sci. 2014, 28, 37–41. [Google Scholar]
- Jahaniaval, F.; Kakuda, Y.; Macrone, M.F. Fatty ccid and triacylglycerol compositions of seed oils of five Amaranthus accessions and their comparison to other oils. J. Am. Oil Chem. Soc. 2000, 77, 847–852. [Google Scholar] [CrossRef]
- Berganza, B.E.; Moran, A.W.; Rodríguez, G.M.; Coto, N.M.; Santamaría, M.; Bressani, R. Effect of variety and location on the total fat, fatty acids and squalene content of amaranth. Plant. Foods Hum. Nutr. 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Gamel, T.H.; Mesallam, A.S.; Damir, A.A.; Shekib, L.A.; Linssen, J.P. Characterization of amaranth seed oils. J. Food Lipids 2007, 14, 323–334. [Google Scholar] [CrossRef]
- Ješko, D.; Čertík, M. Genotype variability of fatty acids in cereal grains. Chem. Listy 2008, 102, 675–677. [Google Scholar]
- Sujak, A.; Dziwulska-Hunek, A. Minerals and fatty acids of amaranth seeds subjected to pre-sowing electromagnetical stimulation. Int. Agrophys. 2010, 24, 375–379. [Google Scholar]
- León-Camacho, M.; García-González, D.L.; Aparicio, R. A detailed and comprehensive study of amaranth (Amaranthus cruentus L.) oil fatty profile. Eur. Food Res. Technol. 2001, 213, 349–355. [Google Scholar] [CrossRef]
- Certik, M.; Shimizu, S. Biosynthesis and regulation of microbial polyunsaturated fatty acid production. J. Biosci. Bioeng. 1999, 87, 1–14. [Google Scholar] [CrossRef]


| Year | A. cruentus L. | A. hypochondriacus x A. hybridus | ± SE | |||||
|---|---|---|---|---|---|---|---|---|
| ‘Pribina’ | Ficha | Aztec | ‘Zobor’ | K-433 | Plainsman | Koniz | ||
| 2016 | 0.97 a | 0.87 b | 0.73 e | 0.83 c | 0.82 d | 0.72 f | 0.71 f | 0.81 ± 0.09 |
| 2017 | 0.95 a | 0.84 c | 0.78 d | 0.86 b | 0.84 c | 0.73 e | 0.69 f | 0.81 ± 0.09 |
| 2018 | 0.90 b | 0.77 e | 0.82 d | 0.84 c | 0.77 e | 0.68 f | 0.68 f | 0.78 ± 0.08 |
| Amino Acid | A. cruentus L. | A. hypochondriacus x A. hybridus | ||||
|---|---|---|---|---|---|---|
| ’Pribina’ | Ficha | ’Zobor’ | K-433 | Plainsman | Koniz | |
| Essential Amino Acid | ||||||
| Arginine (Arg) | 6.16 ± 0.34 b | 6.23 ± 0.14 ab | 7.66 ± 1.29 a | 7.13 ± 0.01 ab | 6.63 ± 1.55 ab | 7.04 ± 0.45 ab |
| Histidine (His) | 2.37 ± 0.09 c | 2.43 ± 0.47 bc | 2.91 ± 0.45 ab | 3,02 ± 0.17 a | 2.61 ± 0.25 abc | 2.91 ± 0.05 ab |
| Isoleucine (Ile) | 2.15 ± 0.20 a | 2.03 ± 0.33 ab | 2.09 ± 0.04 a | 1.43 ± 0.16 b | 1.96 ± 0.76 ab | 1.61 ± 0.07 ab |
| Leucine (Leu) | 3.94 ± 0.42 a | 3.97 ± 0.42 a | 4.71 ± 0.65 a | 4.32 ± 0.19 a | 4.36 ± 0.71 a | 4.47 ± 0.03 a |
| Methionine (Met) | 2.00 ± 0.24 a | 2.18 ± 0.40 a | 1.83 ± 0.01 a | 2.23 ± 0.46 a | 2.13 ± 0.32 a | 2.13 ± 0.04 a |
| Phenylalanine (Phe) | 2.74 ± 0.34 a | 2.79 ± 0.64 a | 3.35 ± 0.38 a | 2.85 ± 0.21 a | 3.21 ±0.64 a | 3.20 ± 0.12 a |
| Threonine (Thr) | 3.06 ± 0.19 a | 2.99 ± 0.42 a | 3.41 ± 0.32 a | 2.92 ± 0.09 a | 3.10 ± 0.75 a | 2.98 ± 0.10 a |
| Lysine (Lys) | 4.23 ± 0.31 a | 4.22 ± 0.13 a | 4.70 ± 0.55 a | 4.30 ± 0.07 a | 4.26 ± 0.92 a | 4.25 ± 0.18 a |
| Valine (Val) | 2.40 ± 0.29 a | 2.22 ± 0.36 abc | 2.30 ± 0.05 ab | 1.63 ± 0.14 c | 2.09 ± 0.72 abc | 1.74 ± 0.09 bc |
| Nonessential Amino Acid | ||||||
| Alanine (Ala) | 2.91 ± 0.16 b | 3.12 ± 0.08 ab | 3.79 ± 0.85 a | 3.14 ± 0.27 ab | 3.04 ± 0.46 b | 3.32 ± 0.14 ab |
| Aspartic acid (Asp) | 6.55 ± 0.13 a | 6.60 ± 0.08 a | 7.53 ± 1.20 a | 7.09 ± 0.11 a | 6.96 ± 0.94 a | 7.17 ± 0.23 a |
| Cysteine (Cys) | 1.78 ± 0.13 b | 2.13 ± 0.06 a | 1.73 ± 0.13 b | 2.10 ± 0.04 a | 2.01 ± 0.13 a | 2.07 ± 0.03 a |
| Glutamic acid (Glu) | 14.85 ± 0.61 a | 16.25 ± 0.80 a | 17.56 ± 1.87 a | 16.36 ± 0.15 a | 17.17 ± 3.35 a | 16.84 ± 0.54 a |
| Glycine (Gly) | 5.74 ± 0.13 b | 6.14 ± 0.21 ab | 6.69 ± 0.94 a | 6.78 ±0.30 a | 6.22 ± 0.76 ab | 6.69 ± 0.10 a |
| Pro (Proline) | 3.12 ± 0.22 a | 3.42 ± 0.11 a | 3.67 ± 0.34 a | 3.34 ± 0.02 a | 3.10 ± 0.99 a | 3.42 ± 0.11 a |
| Ser (Serine) | 5.25 ± 0.07 ab | 5.14 ± 0.17 b | 5.99 ± 0.96 a | 5.88 ± 0.03 ab | 5.80 ± 0.57 ab | 5.84 ± 0.13 ab |
| Tyr (Tyrosine) | 2.39 ± 0.07 ab | 2.71 ± 0.13 a | 2.75 ± 0.30 a | 2.11 ± 0.27 b | 2.71 ± 0.57 a | 2.47 ± 0.13 ab |
| A. cruentus L. | A. hypochondriacus x A. hybridus | ||||||
|---|---|---|---|---|---|---|---|
| ’Pribina’ | Ficha | Aztec | ’Zobor’ | K-433 | Plainsman | Koniz | |
| Oil | 5.42 ± 0.32 a | 5.41 ± 0.56 a | 4.86 ± 0.51 b | 5.55 ± 0.39 a | 5.41 ± 0.23 a | 4.57 ± 0.29 c | 4.82 ± 0.45 b |
| Squalene in oil | 6.94 ± 0.70 a | 6.42 ± 0.47 ab | 3.85 ± 1.20 d | 6.47 ± 0.83 ab | 5.54 ± 0.67 b | 4.21 ± 0.35 cd | 5.26 ± 1.08 bc |
| Squalene in seeds | 0.38 ± 0.05 a | 0.35 ± 0.02 ab | 0.19 ± 0.08 d | 0.36 ± 0.19 ab | 0.30 ± 0.05 bc | 0.19 ± 0.01d | 0.26 ± 0.07 cd |
| Fatty Acid | A. cruentus L. | A. hypochondriacus x A. hybridus | ||
|---|---|---|---|---|
| ’Pribina’ | Ficha | ’Zobor’ | K-433 | |
| Myristic acid (C14:0) | 0.19 ± 0.00 a | 0.19 ± 0.01 a | 0.17 ± 0.02 b | 0.19 ± 0.01 a |
| Palmitic acid(C16:0) | 19.67 ± 0.48 a | 19.81 ± 0.31 a | 20.14 ± 0.25 a | 19.88 ± 0.63 a |
| Palmitoleic acid (C16:1 cis) | 0.15 ± 0.02 a | 0.15 ± 0.02 a | 0.13 ± 0.02 a | 0.15 ± 0.02 a |
| Stearic acid(C18:0) | 3.41 ± 0.30 a | 3.60 ± 0.16 a | 3.67 ± 0.08 a | 3.36 ± 0.29 a |
| Oleic acid (C 18:1 cis) | 32.55 ± 1.53 a | 33.13 ± 2.95 a | 26.25 ± 3.43 b | 31.52 ± 1.85 a |
| Linoleic acid (C18:2 cis) | 40.49 ± 0.55 b | 39.71 ± 1.83 b | 46.06 ± 4.43 a | 41.41 ± 0.70 b |
| α-Linolenic acid (C18:3 cis) | 0.70 ± 0.02 a | 0.74 ± 0.04 a | 0.75 ± 0.06 a | 0.69 ± 0.04 a |
| Arachidic acid (C20:0) | 0.50 ± 0.26 a | 0.50 ± 0.24 a | 0.48 ± 0.25 a | 0.49 ± 0.26 a |
| Eicosenoic acid (C20:1 cis) | 0.33 ± 0.08 a | 0.34 ± 0.09 a | 0.26 ± 0.02 a | 0.31 ± 0.06 a |
| Saturated | 23.77 ± 0.08 b | 23.95 ± 0.29 b | 24.46 ± 0.08 a | 23.93 ± 0.16 b |
| Unsaturated | 75.07 ± 0.75 a | 75.46 ± 0.26 a | 74.74 ± 0.37 a | 74.71 ± 1.05 a |
| Saturated/Unsaturated | 0.32 ± 0.00 b | 0.32 ± 0.00 b | 0.33 ± 0.00 a | 0.32 ± 0.00 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabóová, M.; Záhorský, M.; Gažo, J.; Geuens, J.; Vermoesen, A.; D’Hondt, E.; Hricová, A. Differences in Seed Weight, Amino Acid, Fatty Acid, Oil, and Squalene Content in γ-Irradiation-Developed and Commercial Amaranth Varieties (Amaranthus spp.). Plants 2020, 9, 1412. https://doi.org/10.3390/plants9111412
Szabóová M, Záhorský M, Gažo J, Geuens J, Vermoesen A, D’Hondt E, Hricová A. Differences in Seed Weight, Amino Acid, Fatty Acid, Oil, and Squalene Content in γ-Irradiation-Developed and Commercial Amaranth Varieties (Amaranthus spp.). Plants. 2020; 9(11):1412. https://doi.org/10.3390/plants9111412
Chicago/Turabian StyleSzabóová, Monika, Michal Záhorský, Ján Gažo, Jeroen Geuens, Ann Vermoesen, Els D’Hondt, and Andrea Hricová. 2020. "Differences in Seed Weight, Amino Acid, Fatty Acid, Oil, and Squalene Content in γ-Irradiation-Developed and Commercial Amaranth Varieties (Amaranthus spp.)" Plants 9, no. 11: 1412. https://doi.org/10.3390/plants9111412






