Optimal Ventilation Design for Flammable Gas Leaking from Gas Box Used in Semiconductor Manufacturing: Case Study on Korean Semiconductor Industry
Abstract
:1. Introduction
2. Experimental Setup
2.1. Calculation of Release Rate of Hazardous Substances
2.1.1. KS C IEC 60079-10-1: Release Rate Equation for Hydrogen
2.1.2. SEMI S6-0707E: Release Rate Equation for Hydrogen
2.1.3. SEMI F-15: Release Rate Equation for Hydrogen
2.1.4. Release Rate Calculation Result for Hydrogen
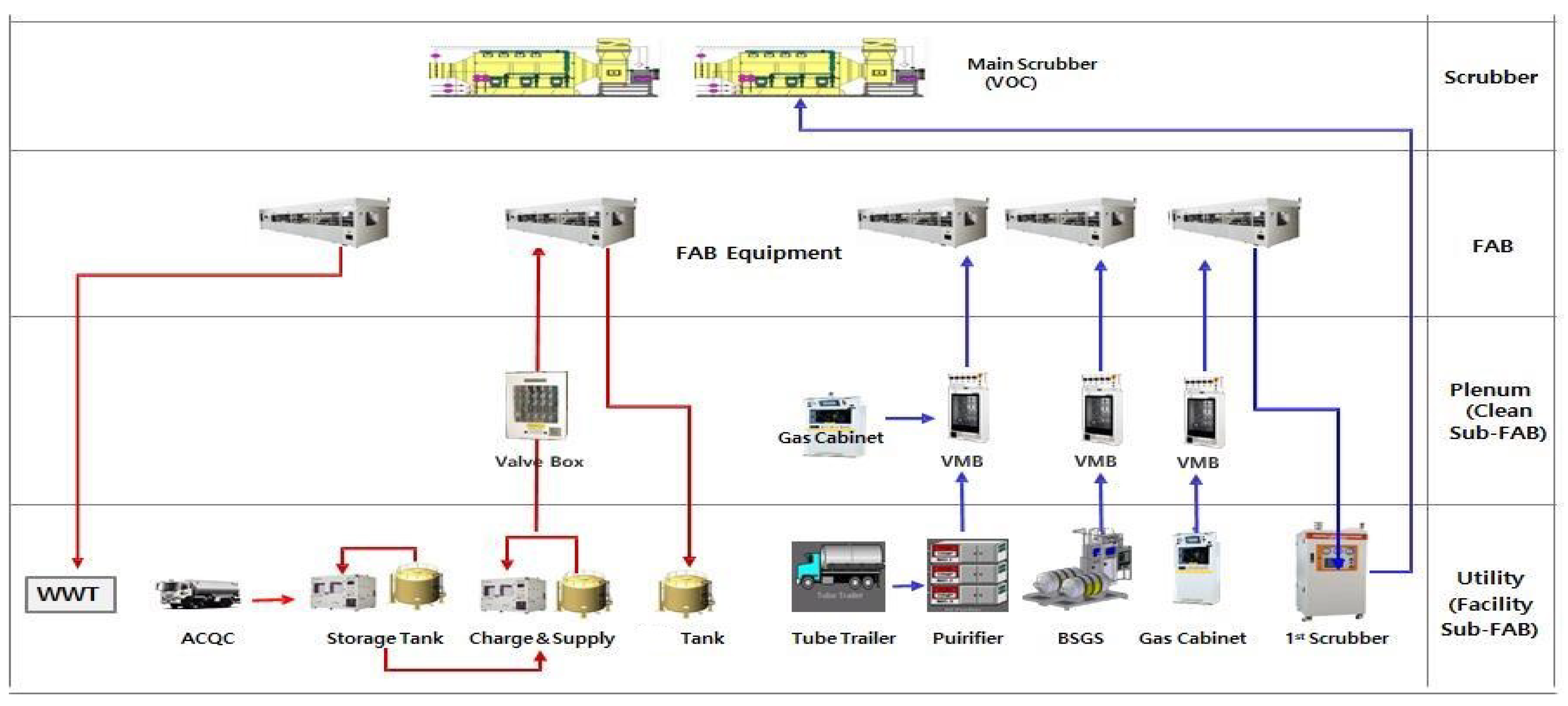
2.2. Gas Box Exhaust System Design Criteria and Selection of Experimental Gas
2.3. Gas Box Modeling
2.4. Duct Diameter Selection
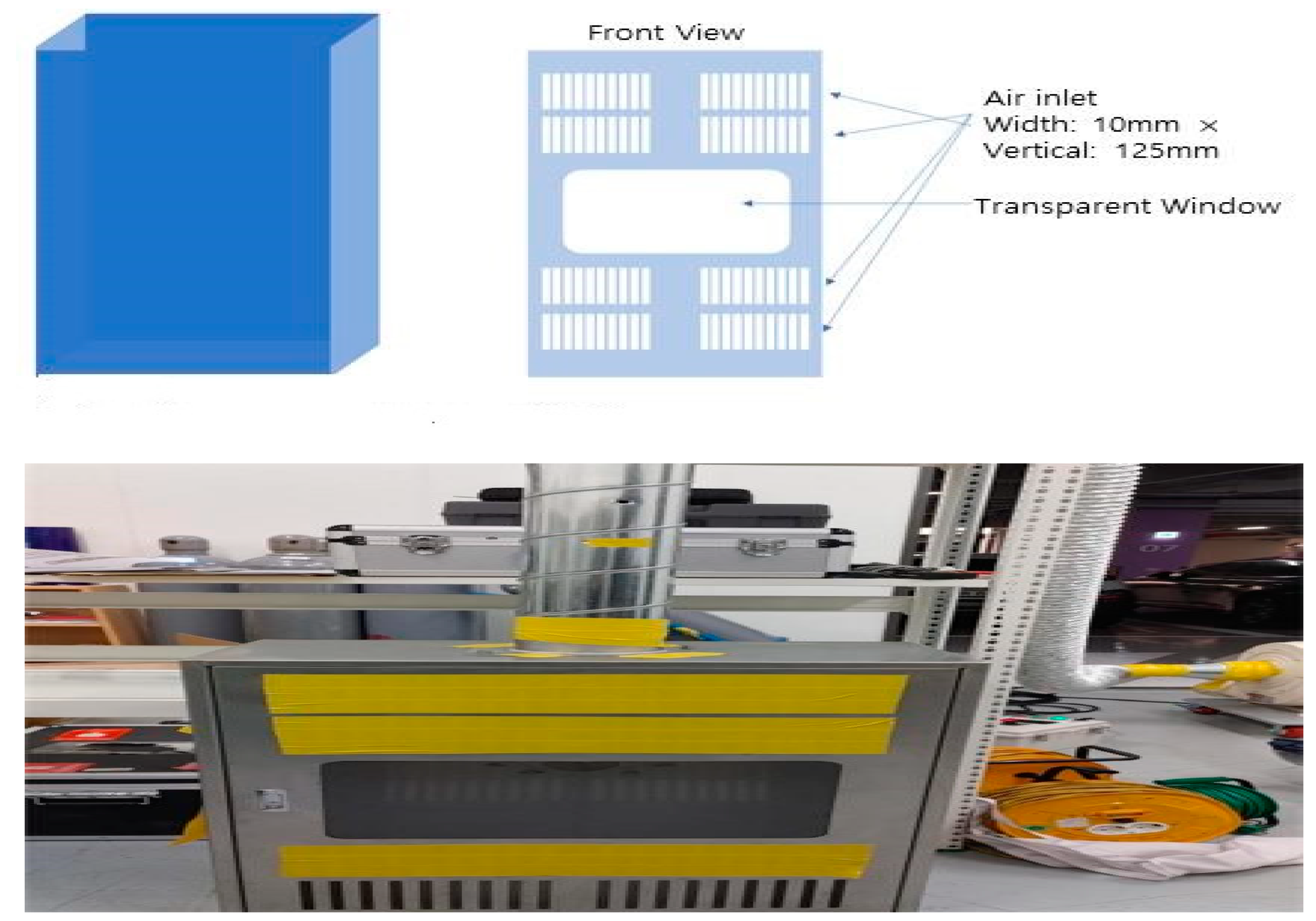
2.5. Air Inlet Location Selection
3. Results
3.1. Duct Transfer Speed Results According to Duct Size
3.1.1. Results for 150 mm (0.15 m) Duct Experiment
3.1.2. Experimental Results for 75 mm (0.75 m) Duct
3.2. Flammable Gas Concentration Measurement Results According to Air Inlet Location
3.2.1. Concentration inside Gas Box with Opening Area of 0% of Duct and Differential Pressure of 192 Pa
3.2.2. Concentration inside Gas Box with 57% Duct Opening Area and Differential Pressure of 98 Pa
3.2.3. Concentration inside Gas Box with 57% Duct Opening Area (Both Openings) and Differential Pressure of 98 Pa
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreaviations (Alphabetical Order)
| CVD | Chemical Vapor Deposition |
| ERC | Equivalent Release Concentration |
| EHS | Environmental, Health, Safety |
| FAB | Fabrication |
| LEL | Low Explosion Limit |
| LPM | Liters Per Minute |
| SEMI | Semiconductor Equipment and Materials International |
| UEL | Upper Explosion Limit |
References
- Eckerman, I. The Bhopal Saga—Causes and Consequences of the World’s Largest Industrial Disaster; Universities Press: Hyderabad, India, 2005. [Google Scholar]
- Lee, K.; Kwon, H.; Cho, S.; Kim, J.; Moon, I. Improvements of safety management system in Korean chemical industry after a large chemical accident. J. Loss Prev. Process Ind. 2016, 42, 6–13. [Google Scholar] [CrossRef]
- Lee, K.; Lee, C. Evaluation of a mitigation system for leakage accidents using mathematical modeling. J. Korean Chem. Eng. 2018, 35, 348–354. [Google Scholar] [CrossRef]
- Korea Ministry of Government Legislation. Occupational Safety and Health Act and Enforcement Regulations of the Act; Korea Ministry of Government Legislation: Sejong, Republic of Korea, 2021.
- Korea Ministry of Government Legislation. Enforcement Decree of the Occupational Safety and Health Act; Korea Ministry of Government Legislation: Sejong, Republic of Korea, 2021.
- Korea Ministry of Government Legislation. Rules on Occupational Safety and Health Act Standards; Korea Ministry of Government Legislation: Sejong, Republic of Korea, 2021.
- Kim, J.D. A Study on the Gas Leak Flow Analysis and Damage Prevention Measures in Specialty Gas Supply Equipment for Semiconductors. Ph.D. Thesis, Korea National University of Transportation, Chungju City, Republic of Korea, 2022. [Google Scholar]
- Liam, Y.H.; Tsung-Ju, H. A Throughput Management System for Semiconductor Wafer Fabrication Facilities: Design, Systems and Implementation. Processes 2018, 6, 16. [Google Scholar] [CrossRef]
- Kim, J.D.; Kwon, K.S. A Study on flow analysis according to the cause of gas leakage in the specialty gas supply device for semiconductors. J. Korean Inst. Gas 2021, 25, 42–52. [Google Scholar] [CrossRef]
- Oh, K.S.; Jeong, E.T.; Shim, W.B.; Baek, J.B. The Effectiveness of Pressure Safety Valves in Chemical Supply Systems to Prevent Explosion, and Overpressure in the Korean Semiconductor Industry. Fire 2023, 6, 344. [Google Scholar] [CrossRef]
- SEMI S6-0707E; EHS Guideline for Exhaust Ventilation of Semiconductor Manufacturing Equipment. Semiconductor Equipment and Materials International: San Jose, CA, USA, 2009.
- KOSHA Guide P-166; Technical Guidelines for Gas Leak Detector Installation and Maintenance. Korean Occupational Safety and Health Agency: Ulsan, Republic of Korea, 2020.
- Kim, S.E.; Lee, K.H.; Kang, C.K.; Jung, S.H. Tracer Gas Test and CFD Analysis of Semiconductor Gas Box for Flammable Gas Leakage. Energies 2023, 15, 8166. [Google Scholar] [CrossRef]
- Linde Publication MSDS; MSDS: Seoul, Republic of Korea, 2011.
- Wu, M.S.; Lee, C.J. A Study for Key Points of PSM to Guarantee the Safety of Liquified Hydrogen Storage Tank. J. Korean Chem. Eng. 2023, 61, 74–79. [Google Scholar] [CrossRef]
- KS C IEC 60079-10-1; Explosive Atmospheres—Part 10-1: Classification of Areas—Explosive Gas Atmospheres. Korean Standard: Eumseong, Republic of Korea, 2017.
- NFPA 497; Recommended Practice for the Classification of Flammable Liquids, Gases, or Vapors and of Hazardous (Classified) Locations for Electrical Installations in Chemical Process Areas. National Fire Protection Association: Quincy, MA, USA, 2017.
- API R.P. 505; Recommended Practice for Classification of Locations for Electrical Installations at Petroleum Facilities Classified as Class I, Zone 0, Zone 1, and Zone 2. API Publishing Services: Washington, DC, USA, 2012.
- Seo, M.S.; Kim, K.S.; Hwang, Y.W.; Chun, Y.W. A Study on Determination of Range of Hazardous Area Caused by the Secondary Grade of Release of Vapor Substances Considering Material Characteristic and Operating Condition. J. Korean Inst. Gas 2018, 22, 13–26. [Google Scholar] [CrossRef]
- Kim, S.G. A Study on the Optimal Exhaust Ventilation Ddesign for Gas Panel of Semiconductor Manufacturing Equipment. Ph.D. Thesis, Korea National University of Transportation, Chungju City, Republic of Korea, 2022. [Google Scholar]
- Park, C.; Kim, C. Risk assessment of Explosion of Mixed Dust Generated in Semiconductor Manufacturing. J. Korean Inst. Electr. 2018, 67, 474–478. [Google Scholar] [CrossRef]
- Ngai, E.Y.; Huang, K.P.-P.; Chen, J.-R.; Shen, C.-C.; Tsai, H.-Y.; Chen, S.-K.; Hu, S.-C.; Yeh, P.-Y.; Liu, C.-D.; Chang, Y.-Y.; et al. Field tests of release, ignition, and explosion from silane cylinder valve and gas cabinet. Process Saf. Prog. 2007, 26, 265–282. [Google Scholar] [CrossRef]
- Yet-Pole, I.; Chiu, Y.-L.; Wu, S.-J. The simulation of air recirculation and fire/explosion phenomena within a semiconductor factory. J. Hazard. Mater. 2009, 163, 1040–1051. [Google Scholar] [CrossRef]
- Kim, J.D.; Han, S.A.; Yang, W.-B.; Rhim, J.-G. A study on the Internal Flow Analysis of Gas Cylinder Cabinet for Specialty Gas of Semiconductor. J. Korean Inst. Gas. 2020, 24, 74–81. [Google Scholar] [CrossRef]
- Kim, C.K. Experimental Study on the Safety of a Valve for a Special Gas Cylinder. J. Korean Inst. Gas 2013, 17, 14–19. [Google Scholar] [CrossRef]
- IEC 60079-10-1; Explosive Atmospheres—Part 10-1: Classification of Areas—Explosive Gas Atmospheres. International Electrotechnical Commission: Geneva, Switzerland, 2013.
- KOSHA. GUIDE E-150. Technical Guidelines for Establishing Gas Explosion Hazard Locations; Korea Occupational Safety and Health Agency: Ulsan, Republic of Korea, 2017. [Google Scholar]
- Kim, S.R.; Lim, K.Y.; Yang, W.-B.; Rhim, J.-G. A Study on the Explosion Hazardous Area in the Secondary Leakage of Vapor Phase Materials Based on the Test Results and the Leak Rate According to SEMI S6 in the Semiconductor Industry. J. Korean Inst. Gas 2020, 24, 15–21. [Google Scholar] [CrossRef]
- SEMI S2; Environmental, Health, and Safety Guideline for Semiconductor Manufacturing Equipment. Semiconductor Equipment and Materials International: San Jose, CA, USA, 2016.
- SEMI F15-93; Test Method for Enclosure Using Sulfur Hexafluoride Tracer Gas and Gas Chromatography. Semiconductor Equipment and Materials International: San Jose, CA, USA, 2004.
- Byun, Y.S. A Study on Safety Improvement for Mobile Hydrogen Refueling Station by HAZOP Analysis. J. Korean Inst. Hyd. 2021, 32, 299–307. [Google Scholar] [CrossRef]
- Kim, H.R.; Kang, S.K. Analysis of Damage Range and Impact of On-Site Hydrogen Fueling Station Using Quantitative Risk Assessment Program (Hy-KoRAM). Anal. J. Korean Inst. Hyd. 2020, 31, 459–466. [Google Scholar] [CrossRef]





| Sign | Meaning |
|---|---|
| Critical pressure (Pa) | |
| Atmospheric pressure (Pa) | |
| P | Internal pressure (Pa) |
| γ | Polytropic index (dimensionless) |
| Discharge coefficient (dimensionless) | |
| Mass leak rate (kg/s) | |
| R | Ideal gas constant (8314 J/kmol·k) |
| S | |
| Z | Compressibility factor (dimensionless) |
| T | Absolute temperature (K) |
| M | Molecular weight (kg/kmol) |
| Sign | Meaning |
|---|---|
| Density of gas flowing through straight tube at downstream (ambient) condition (g/cm3) | |
| Density of gas flowing through straight tube at upstream condition (g/cm3) | |
| 0.02 surface roughness parameter for smooth pipe (dimensionless) | |
| γ | Polytropic index (dimensionless) |
| Mass flow rate of gas flowing in straight tube (kg/s) | |
| Square of upstream Mach number (dimensionless) | |
| Upstream absolute pressure (pa) | |
| Downstream absolute pressure (pa) | |
| L | Pipe length (m) |
| D | Pipe diameter (m) |
| Q | Volume flow rate of gas (L/min) |
| V | 22.4 (L/mole) molar ideal gas volume |
| M | Molecular weight (kg/kmol) |
| S |
| Calculation Source | Pressure (Pa(a)) | Release Opening | Volume Flow Rate of Gas (Liters per Minute) |
|---|---|---|---|
| KS C IEC 60079-10-1 | 377,143 | 0.25 | 32 |
| SEMI S6-0707E | 377,143 | 31.65 | 3146 |
| SEMI F-15 | 377,143 | - | 28 |
| Air Inlet | Opening Area | Internal Conveyance Speed of Duct | Number of Ventilation Times per Minute | Differential Pressure (Pa) | ||
|---|---|---|---|---|---|---|
| Horizontal Length (mm) | Vertical Length (mm) | Number of Air Inlets | ||||
| 10 | 125 | 0 | 0 | 1 | 5.05 | 65 |
| 10 | 125 | 1 | 1250 | 1.5 | 7.57 | 57 |
| 10 | 125 | 2 | 1250 | 1.8 | 9.09 | 40 |
| 10 | 125 | 3 | 3750 | 2 | 10.10 | 26 |
| 10 | 125 | 4 | 5000 | 2.1 | 10.60 | 18.6 |
| 10 | 125 | 5 | 6250 | 2.15 | 10.86 | 13.7 |
| 10 | 125 | 6 | 7500 | 2.2 | 11.11 | 10 |
| 10 | 125 | 7 | 8750 | 2.25 | 11.36 | 8 |
| Air Inlet | Opening Area | Internal Conveyance Speed of Duct | Number of Ventilation Times per Minute | Differential Pressure (Pa) | ||
|---|---|---|---|---|---|---|
| Horizontal Length (m) | Vertical Length (m) | Number of Air Inlets | ||||
| 10 | 125 | 0 | 0 | 4.1 | 5.18 | 192 |
| 10 | 125 | 0.5 | 625 | 4.9 | 6.19 | 150 |
| 10 | 125 | 1 | 1250 | 6.4 | 8.08 | 146 |
| 10 | 125 | 1.5 | 1875 | 6.8 | 8.58 | 138 |
| 10 | 125 | 2 | 2500 | 7 | 8.84 | 98 |
| 10 | 125 | 2.5 | 3125 | 7.5 | 9.47 | 81 |
| 10 | 125 | 3 | 3750 | 8 | 10.10 | 70 |
| 10 | 125 | 3.5 | 4375 | 8 | 10.10 | 70 |
| Sampling Point | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Measured value (ppm) | 119.41 | 129.41 | 211.33 | 200.43 | 109.22 | 134.89 |
| Equivalent concentration (ppm) | 11,941 | 12,941 | 21,333 | 20,043 | 10,922 | 13,489 |
| Reference concentration (ppm) | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 |
| % LEL | 29.85 | 32.35 | 52.83 | 50.11 | 27.30 | 33.72 |
| Pass/Fail | Fail | Fail | Fail | Fail | Fail | Fail |
| Sampling Point | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Measured value (ppm) | 81.27 | 105.76 | 102.52 | 69.39 | 82.10 | 104.64 |
| Equivalent concentration (ppm) | 8127 | 10,576 | 10,252 | 6939 | 8210 | 10,464 |
| Reference concentration (ppm) | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 |
| % LEL | 20.32 | 26.44 | 25.63 | 17.35 | 20.53 | 26.16 |
| Pass/Fail | Pass | Fail | Fail | Pass | Pass | Fail |
| Sampling Point | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Measured value (ppm) | 81.95 | 90.90 | 77.18 | 71.42 | 80.35 | 91.11 |
| Equivalent concentration (ppm) | 8195 | 9090 | 7718 | 7142 | 8035 | 9111 |
| Reference concentration (ppm) | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 |
| % LEL | 20.49 | 22.73 | 19.30 | 17.86 | 20.09 | 22.78 |
| Pass/Fail | Pass | Pass | Pass | Pass | Pass | Pass |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-R.; Moon, H.-S.; Jeong, P.-H. Optimal Ventilation Design for Flammable Gas Leaking from Gas Box Used in Semiconductor Manufacturing: Case Study on Korean Semiconductor Industry. Fire 2023, 6, 432. https://doi.org/10.3390/fire6110432
Kim S-R, Moon H-S, Jeong P-H. Optimal Ventilation Design for Flammable Gas Leaking from Gas Box Used in Semiconductor Manufacturing: Case Study on Korean Semiconductor Industry. Fire. 2023; 6(11):432. https://doi.org/10.3390/fire6110432
Chicago/Turabian StyleKim, Sang-Ryung, Hyo-Shik Moon, and Phil-Hoon Jeong. 2023. "Optimal Ventilation Design for Flammable Gas Leaking from Gas Box Used in Semiconductor Manufacturing: Case Study on Korean Semiconductor Industry" Fire 6, no. 11: 432. https://doi.org/10.3390/fire6110432
APA StyleKim, S.-R., Moon, H.-S., & Jeong, P.-H. (2023). Optimal Ventilation Design for Flammable Gas Leaking from Gas Box Used in Semiconductor Manufacturing: Case Study on Korean Semiconductor Industry. Fire, 6(11), 432. https://doi.org/10.3390/fire6110432





