Recent Advances in Pancreatic Ductal Adenocarcinoma
Share This Topical Collection
Editors
Topical Collection Information
Dear Colleagues,
We would like to invite you to submit either original research or review articles for this Collection on “Recent Advances in Pancreatic Ductal Adenocarcinoma (PDAC)”.
This Collection will focus on the recent advances in PDAC research. The topics will include but are not limited to advances in (1) pancreatic cancer surgery, especially around the equipoise of laparoscopic/robotic vs. open resections, arterial resections, and surgical management of stage 4 PDAC; (2) diagnostic and prognostic biomarkers in blood, urine, and/or tissues; (3) the pancreatic tumour microenvironment; (4) chemotherapy in neoadjuvant, adjuvant, and palliative settings; (5) radiotherapy or radio/chemotherapy; (6) postsurgical complications; (7) novel targeted therapies; (8) immunotherapy; and (9) pancreatic tumour microbiome.
Thank you for your consideration.
Kind regards,
Dr. Sumit Sahni
Dr. Anubhav Mittal
Prof. Dr. Jaswinder Samra
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- pancreatic ductal adenocarcinoma
- pancreatoduodenectomy
- distal pancreatectomy
- neoadjuvant chemotherapy
- biomarkers
- postsurgical complications
- targeted therapies
- tumour microenvironment
Related Special Issue
Published Papers (14 papers)
Open AccessReview
Tumor Characteristics and Clinical Features of the Patient as Prognostic Factors in PDAC
by
Karina Udrycka, Kamil Rutkowski, Anton Osnytskyy and Ewa Małecka-Wojciesko
Viewed by 311
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of cancer-related deaths among individuals over the age of 50. It is characterized by exceptional aggressiveness and is often diagnosed at an advanced stage, highlighting the importance of assessing prognostic factors. The deep awareness
[...] Read more.
Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of cancer-related deaths among individuals over the age of 50. It is characterized by exceptional aggressiveness and is often diagnosed at an advanced stage, highlighting the importance of assessing prognostic factors. The deep awareness of these factors may help in better prevention and treatment planning, eventually improving the outcomes. Favorable prognostic factors include female gender, low tumor stage, ECOG (Eastern Cooperative Oncology Group) 0–1, lowest ASI (Activated Stroma Index), low-grade tumor budding, age below 40, adequate nutrition, and absence of distant metastases. Conversely, unfavorable prognostic factors include the presence of distant metastases and metastases into lymph nodes, high tumor stage, LVI (lymphovascular invasion), PNI (perineural invasion), tumor size above 3 cm, invasion into vessels, higher G grade, higher ASI, high-grade tumor budding, more than 1 CTC (circulating tumor cells) in the bloodstream, ECOG 3–4, age above 40, Black ethnicity, malnutrition, and sarcopenia. This review discusses the prognostic factors of PDAC related to tumor characteristics and the patient’s clinical issues. The aim of this review is to synthesize current evidence on prognostic determinants in PDAC, with particular attention to both tumor characteristics and patient-specific clinical features. To achieve this, a comprehensive literature review was performed using PubMed, BrowZine Library, Cochrane Library, SpringerLink, Wiley Online Library, BMJ Journals, and Google Scholar. Relevant studies addressing established and emerging prognostic markers were critically analyzed to provide an updated overview of factors that may influence survival and treatment outcomes. By integrating available data, this review seeks not only to summarize classical prognostic variables but also to highlight novel and underrecognized markers that may hold future clinical relevance. Such an approach may contribute to the refinement of prognostic models, support more accurate patient counseling, and ultimately aid in the optimization of therapeutic strategies for individuals affected by PDAC.
Full article
►▼
Show Figures
Open AccessArticle
Repeated COVID-19 Vaccination as a Poor Prognostic Factor in Pancreatic Cancer: A Retrospective, Single-Center Cohort Study
by
Makoto Abue, Mai Mochizuki, Rie Shibuya-Takahashi, Kensuke Ota, Yuta Wakui, Wataru Iwai, Jun Kusaka, Masashi Saito, Shinichi Suzuki, Ikuro Sato and Keiichi Tamai
Cited by 2 | Viewed by 38804
Abstract
Background/Objectives: The COVID-19 vaccine is a significant technological advancement with widespread global use. However, its effect on cancer immunity, particularly with repeated vaccinations, remains unclear. We aimed to investigate the relationship between repeated vaccinations and pancreatic cancer (PC) prognosis. Additionally, we examined
[...] Read more.
Background/Objectives: The COVID-19 vaccine is a significant technological advancement with widespread global use. However, its effect on cancer immunity, particularly with repeated vaccinations, remains unclear. We aimed to investigate the relationship between repeated vaccinations and pancreatic cancer (PC) prognosis. Additionally, we examined serum IgG4 levels, known to be an immune suppressor which increases with repeated vaccinations.
Methods: We retrospectively examined the effect of vaccination on survival in 272 PC patients diagnosed at our hospital from January 2018 to November 2023 and analyzed prognostic factors, including IgG4 levels in 96 PC patients. Immunohistochemistry for Foxp3 in the tumor tissue was performed, and the serum IgG4 level was measured. Serum samples from 79 patients with benign and malignant diseases, including PC, were collected between September and November 2023, and the spike-specific IgG4 level was determined using an enzyme-linked immunosorbent assay.
Results: The overall survival (OS) of PC patients was shortened in those vaccinated three times or more, and the total serum IgG4 levels increased with the number of vaccinations. Of note, OS was significantly shorter in the high IgG4 group, and Foxp3-positive cells in the tumor tissues were increased. Repeated vaccinations increased the spike-specific IgG4 levels, and a positive correlation was observed between spike-specific IgG4 and the total IgG4.
Conclusions: These findings highlight repeated vaccination as a poor prognostic factor in PC patients and suggest that IgG4 is induced by repeated vaccination and may be associated with a poor prognosis in these patients.
Full article
►▼
Show Figures
Open AccessArticle
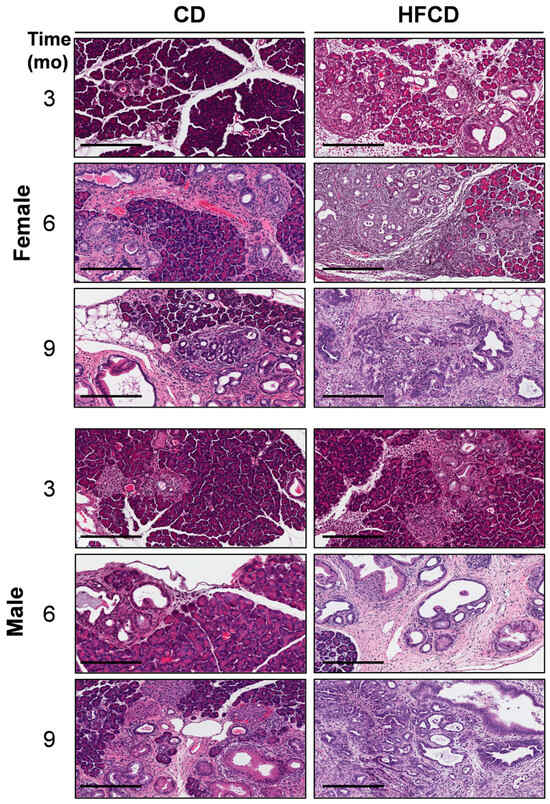
Upregulated Matrisomal Proteins and Extracellular Matrix Mechanosignaling Underlie Obesity-Associated Promotion of Pancreatic Ductal Adenocarcinoma
by
Richard T. Waldron, Aurelia Lugea, Hui-Hua Chang, Hsin-Yuan Su, Crystal Quiros, Michael S. Lewis, Mingtian Che, V. Krishnan Ramanujan, Enrique Rozengurt, Guido Eibl and Stephen J. Pandol
Cited by 1 | Viewed by 3753
Abstract
Diet-induced obesity (DIO) promotes pancreatic ductal adenocarcinoma (PDAC) in mice expressing KRasG12D in the pancreas (KC mice), but the precise mechanisms remain unclear. Here, we performed multiplex quantitative proteomic and phosphoproteomic analysis by liquid chromatography–tandem mass spectrometry and further bioinformatic and spatial analysis
[...] Read more.
Diet-induced obesity (DIO) promotes pancreatic ductal adenocarcinoma (PDAC) in mice expressing KRasG12D in the pancreas (KC mice), but the precise mechanisms remain unclear. Here, we performed multiplex quantitative proteomic and phosphoproteomic analysis by liquid chromatography–tandem mass spectrometry and further bioinformatic and spatial analysis of pancreas tissues from control-fed versus DIO KC mice after 3, 6, and 9 months. Normal pancreatic parenchyma and associated proteins were steadily eliminated and the novel proteins, phosphoproteins, and signaling pathways associated with PDAC tumorigenesis increased until 6 months, when most males exhibited cancer, but females did not. Differentially expressed proteins and phosphoproteins induced by DIO revealed the crucial functional role of matrisomal proteins, which implies the roles of upstream regulation by TGFβ, extracellular matrix-receptor signaling to downstream PI3K-Akt-mTOR-, MAPK-, and Yap/Taz activation, and crucial effects in the tumor microenvironment such as metabolic alterations and signaling crosstalk between immune cells, cancer-associated fibroblasts (CAFs), and tumor cells. Staining tissues from KC mice localized the expression of several prognostic PDAC biomarkers and elucidated tumorigenic features, such as robust macrophage infiltration, acinar–ductal metaplasia, mucinous PanIN, distinct nonmucinous atypical flat lesions (AFLs) surrounded by smooth muscle actin-positive CAFs, invasive tumors with epithelial–mesenchymal transition arising close to AFLs, and expanding deserted areas by 9 months. We next used Nanostring GeoMX to characterize the early spatial distribution of specific immune cell subtypes in distinct normal, stromal, and PanIN areas. Taken together, these data richly contextualize DIO promotion of Kras-driven PDAC tumorigenesis and provide many novel insights into the signaling pathways and processes involved.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceSystematic Review
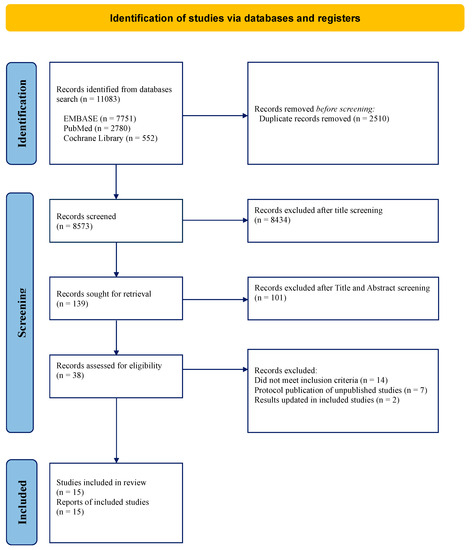
Neo-Adjuvant Treatment in Primary Resectable Pancreatic Cancer: A Systematic Review and PRISMA-Compliant Updated Metanalysis of Oncological Outcomes
by
Raffaello Roesel, Letizia Deantonio, Lorenzo Bernardi, Maria Luisa Garo, Pietro Majno-Hurst, Alberto Vannelli, Marco Cefalì, Maria Celeste Palmarocchi, Maria Carla Valli, Guido Pesola, Alessandra Cristaudi and Sara De Dosso
Cited by 8 | Viewed by 2410
Abstract
Background: Despite advances in treatment, the prognosis of resectable pancreatic adenocarcinoma remains poor. Neoadjuvant therapy (NAT) has gained great interest in hopes of improving survival. However, the results of available studies based on different treatment approaches, such as chemotherapy and chemoradiotherapy, showed contrasting
[...] Read more.
Background: Despite advances in treatment, the prognosis of resectable pancreatic adenocarcinoma remains poor. Neoadjuvant therapy (NAT) has gained great interest in hopes of improving survival. However, the results of available studies based on different treatment approaches, such as chemotherapy and chemoradiotherapy, showed contrasting results. The aim of this systematic review and meta-analysis is to clarify the benefit of NAT compared to upfront surgery (US) in primarily resectable pancreatic adenocarcinoma. Methods: A PRISMA literature review identified 139 studies, of which 15 were finally included in the systematic review and meta-analysis. All data from eligible articles was summarized in a systematic summary and then used for the meta-analysis. Specifically, we used HR for OS and DFS and risk estimates (odds ratios) for the R0 resection rate and the N+ rate. The risk of bias was correctly assessed according to the nature of the studies included. Results: From the pooled HRs, OS for NAT patients was better, with an HR for death of 0.80 (95% CI: 0.72–0.90) at a significance level of less than 1%. In the sub-group analysis, no difference was found between patients treated with chemoradiotherapy or chemotherapy exclusively. The meta-analysis of seven studies that reported DFS for NAT resulted in a pooled HR for progression of 0.66 (95% CI: 0.56–0.79) with a significance level of less than 1%. A significantly lower risk of positive lymph nodes (OR: 0.45; 95% CI: 0.32–0.63) and an improved R0 resection rate (OR: 1.70; 95% CI: 1.23–2.36) were also found in patients treated with NAT, despite high heterogeneity. Conclusions: NAT is associated with improved survival for patients with resectable pancreatic adenocarcinoma; however, the optimal treatment strategy has yet to be defined, and further studies are required.
Full article
►▼
Show Figures
Open AccessReview
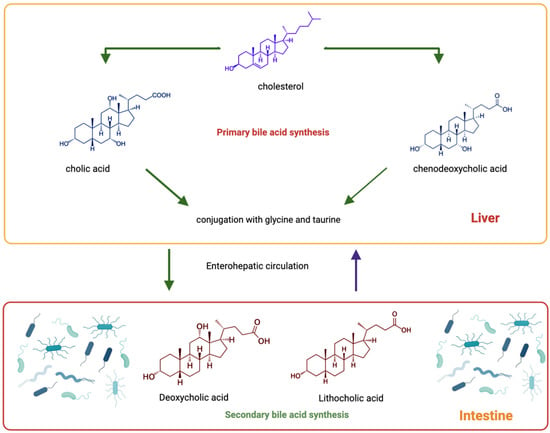
Bile Acids and Microbiota Interplay in Pancreatic Cancer
by
Pratibha Malhotra, Ranjith Palanisamy, Jose A. Caparros-Martin and Marco Falasca
Cited by 14 | Viewed by 5849
Abstract
Evidence suggests the involvement of the microbiota, including oral, intra-tumoral and gut, in pancreatic cancer progression and response to therapy. The gut microbiota modulates the bile acid pool and is associated with maintaining host physiology. Studies have shown that the bile acid/gut microbiota
[...] Read more.
Evidence suggests the involvement of the microbiota, including oral, intra-tumoral and gut, in pancreatic cancer progression and response to therapy. The gut microbiota modulates the bile acid pool and is associated with maintaining host physiology. Studies have shown that the bile acid/gut microbiota axis is dysregulated in pancreatic cancer. Bile acid receptor expression and bile acid levels are dysregulated in pancreatic cancer as well. Studies have also shown that bile acids can cause pancreatic cell injury and facilitate cancer cell proliferation. The microbiota and its metabolites, including bile acids, are also altered in other conditions considered risk factors for pancreatic cancer development and can alter responses to chemotherapeutic treatments, thus affecting patient outcomes. Altogether, these findings suggest that the gut microbial and/or bile acid profiles could also serve as biomarkers for pancreatic cancer detection. This review will discuss the current knowledge on the interaction between gut microbiota interaction and bile acid metabolism in pancreatic cancer.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
PDAC, the Influencer Cancer: Cross-Talk with Tumor Microenvironment and Connected Potential Therapy Strategies
by
Leonardo Mercanti, Maria Sindaco, Mariangela Mazzone, Maria Carmela Di Marcantonio, Mariagrazia Piscione, Raffaella Muraro and Gabriella Mincione
Cited by 32 | Viewed by 7396
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is among the leading causes of death by cancer in the world. What makes this pathological condition particularly lethal is a combination of clinical and molecular heterogeneity, lack of early diagnostic indexes, and underwhelming results from current therapeutic protocols.
[...] Read more.
Pancreatic ductal adenocarcinoma (PDAC) is among the leading causes of death by cancer in the world. What makes this pathological condition particularly lethal is a combination of clinical and molecular heterogeneity, lack of early diagnostic indexes, and underwhelming results from current therapeutic protocols. A major cause of PDAC chemoresistance seems to lie in the ability of cancer cells to spread out and fill the pancreatic parenchyma, exchanging nutrients, substrates, and even genetic material with cells from the surrounding tumor microenvironment (TME). Several components can be found in the TME ultrastructure, including collagen fibers, cancer-associated fibroblasts, macrophages, neutrophils, mast cells, and lymphocytes. Cross-talk between PDAC and TME cells results in the latter being converted into cancer-favoring phenotypes; this behavior could be compared to an influencer guiding followers into supporting his activity. Moreover, TME could be a potential target for some of the newest therapeutic strategies; these include the use of pegvorhyaluronidase-α and CAR-T lymphocytes against HER2, FAP, CEA, MLSN, PSCA, and CD133. Other experimental therapy options are being currently studied, aiming to interfere with the KRAS pathway, DNA-repairing proteins, and apoptosis resistance in PDAC cells. Hopefully these new approaches will grant better clinical outcomes in future patients.
Full article
►▼
Show Figures
Open AccessReview
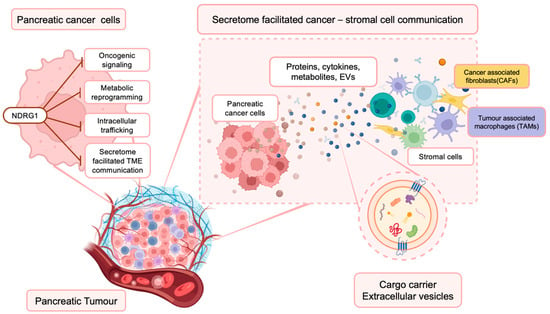
Re-Shaping the Pancreatic Cancer Tumor Microenvironment: A New Role for the Metastasis Suppressor NDRG1
by
Jiawei Chang, Zoe H. Y. Lo, Shafi Alenizi and Zaklina Kovacevic
Cited by 1 | Viewed by 4207
Abstract
Pancreatic cancer (PaC) is a highly aggressive disease, with poor response to current treatments and 5-year survival rates of 10–15%. PaC progression is facilitated by its interaction with the complex and multifaceted tumor microenvironment (TME). In the TME, cancer cells and surrounding stromal
[...] Read more.
Pancreatic cancer (PaC) is a highly aggressive disease, with poor response to current treatments and 5-year survival rates of 10–15%. PaC progression is facilitated by its interaction with the complex and multifaceted tumor microenvironment (TME). In the TME, cancer cells and surrounding stromal cells constantly communicate with each other via the secretion and uptake of factors including cytokines, chemokines, growth factors, metabolites, and extracellular vesicles (EVs), reshaping the landscape of PaC. Recent studies demonstrated that the metastasis suppressor N-myc downstream regulated 1 (NDRG1) not only inhibits oncogenic signaling pathways in PaC cells but also alters the communication between PaC cells and the surrounding stroma. In fact, NDRG1 was found to influence the secretome of PaC cells, alter cancer cell metabolism, and interfere with intracellular trafficking and intercellular communication between PaC cells and surrounding fibroblasts. This review will present recent advancements in understanding the role of NDRG1 in PaC progression, with a focus on how this molecule influences PaC-stroma communication and its potential for re-shaping the PaC TME.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
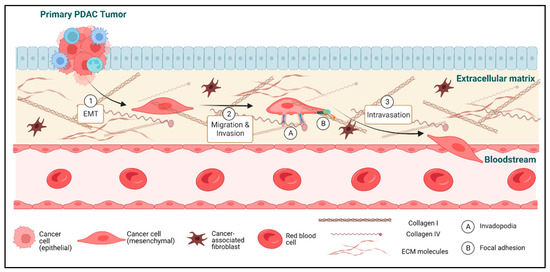
The Cell Biology of Metastatic Invasion in Pancreatic Cancer: Updates and Mechanistic Insights
by
Vidhu B. Joshi, Omar L. Gutierrez Ruiz and Gina L. Razidlo
Cited by 15 | Viewed by 6436
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related mortality worldwide. This is largely due to the lack of routine screening protocols, an absence of symptoms in early-stage disease leading to late detection, and a paucity of effective treatment options.
[...] Read more.
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related mortality worldwide. This is largely due to the lack of routine screening protocols, an absence of symptoms in early-stage disease leading to late detection, and a paucity of effective treatment options. Critically, the majority of patients either present with metastatic disease or rapidly develop metastatic disease. Thus, there is an urgent need to deepen our understanding of metastasis in PDAC. During metastasis, tumor cells escape from the primary tumor, enter the circulation, and travel to a distant site to form a secondary tumor. In order to accomplish this relatively rare event, tumor cells develop an enhanced ability to detach from the primary tumor, migrate into the surrounding matrix, and invade across the basement membrane. In addition, cancer cells interact with the various cell types and matrix proteins that comprise the tumor microenvironment, with some of these factors working to promote metastasis and others working to suppress it. In PDAC, many of these processes are not well understood. The purpose of this review is to highlight recent advances in the cell biology of the early steps of the metastatic cascade in pancreatic cancer. Specifically, we will examine the regulation of epithelial-to-mesenchymal transition (EMT) in PDAC and its requirement for metastasis, summarize our understanding of how PDAC cells invade and degrade the surrounding matrix, and discuss how migration and adhesion dynamics are regulated in PDAC to optimize cancer cell motility. In addition, the role of the tumor microenvironment in PDAC will also be discussed for each of these invasive processes.
Full article
►▼
Show Figures
Open AccessPerspective
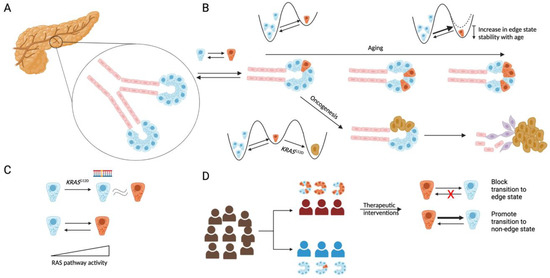
Towards a Synthesis of the Non-Genetic and Genetic Views of Cancer in Understanding Pancreatic Ductal Adenocarcinoma Initiation and Prevention
by
Vishaka Gopalan and Sridhar Hannenhalli
Cited by 1 | Viewed by 2046
Abstract
While much of the research in oncogenesis and cancer therapy has focused on mutations in key cancer driver genes, more recent work suggests a complementary non-genetic paradigm. This paradigm focuses on how transcriptional and phenotypic heterogeneity, even in clonally derived cells, can create
[...] Read more.
While much of the research in oncogenesis and cancer therapy has focused on mutations in key cancer driver genes, more recent work suggests a complementary non-genetic paradigm. This paradigm focuses on how transcriptional and phenotypic heterogeneity, even in clonally derived cells, can create sub-populations associated with oncogenesis, metastasis, and therapy resistance. We discuss this complementary paradigm in the context of pancreatic ductal adenocarcinoma. A better understanding of cellular transcriptional heterogeneity and its association with oncogenesis can lead to more effective therapies that prevent tumor initiation and slow progression.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
Hypoxia, a Targetable Culprit to Counter Pancreatic Cancer Resistance to Therapy
by
Raefa Abou Khouzam, Jean-Marie Lehn, Hemma Mayr, Pierre-Alain Clavien, Michael Bradley Wallace, Michel Ducreux, Perparim Limani and Salem Chouaib
Cited by 16 | Viewed by 5327
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer, and it is a disease of dismal prognosis. While immunotherapy has revolutionized the treatment of various solid tumors, it has achieved little success in PDAC. Hypoxia within the stroma-rich tumor microenvironment
[...] Read more.
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer, and it is a disease of dismal prognosis. While immunotherapy has revolutionized the treatment of various solid tumors, it has achieved little success in PDAC. Hypoxia within the stroma-rich tumor microenvironment is associated with resistance to therapies and promotes angiogenesis, giving rise to a chaotic and leaky vasculature that is inefficient at shuttling oxygen and nutrients. Hypoxia and its downstream effectors have been implicated in immune resistance and could be contributing to the lack of response to immunotherapy experienced by patients with PDAC. Paradoxically, increasing evidence has shown hypoxia to augment genomic instability and mutagenesis in cancer, suggesting that hypoxic tumor cells could have increased production of neoantigens that can potentially enable their clearance by cytotoxic immune cells. Strategies aimed at relieving this condition have been on the rise, and one such approach opts for normalizing the tumor vasculature to reverse hypoxia and its downstream support of tumor pathogenesis. An important consideration for the successful implementation of such strategies in the clinic is that not all PDACs are equally hypoxic, therefore hypoxia-detection approaches should be integrated to enable optimal patient selection for achieving improved patient outcomes.
Full article
►▼
Show Figures
Open AccessReview
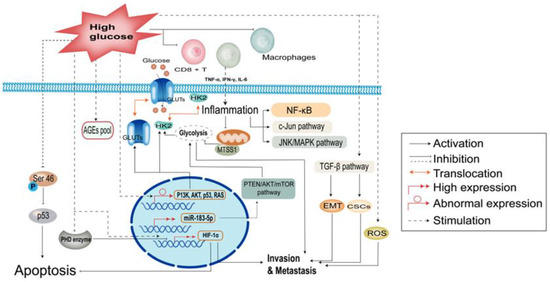
Biological and Clinical Impacts of Glucose Metabolism in Pancreatic Ductal Adenocarcinoma
by
Zhao Liu, Hiromitsu Hayashi, Kazuki Matsumura, Norio Uemura, Yuta Shiraishi, Hiroki Sato and Hideo Baba
Cited by 13 | Viewed by 4154
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a lethal cancer type as it is prone to metastases and is difficult to diagnose at an early stage. Despite advances in molecular detection, its clinical prognosis remains poor and it is expected to become the second leading
[...] Read more.
Pancreatic ductal adenocarcinoma (PDAC) is a lethal cancer type as it is prone to metastases and is difficult to diagnose at an early stage. Despite advances in molecular detection, its clinical prognosis remains poor and it is expected to become the second leading cause of cancer-related deaths. Approximately 85% of patients develop glucose metabolism disorders, most commonly diabetes mellitus, within three years prior to their pancreatic cancer diagnosis. Diabetes, or glucose metabolism disorders related to PDAC, are typically associated with insulin resistance, and beta cell damage, among other factors. From the perspective of molecular regulatory mechanisms, glucose metabolism disorders are closely related to PDAC initiation and development and to late invasion and metastasis. In particular, abnormal glucose metabolism impacts the nutritional status and prognosis of patients with PDAC. Meanwhile, preliminary research has shown that metformin and statins are effective for the prevention or treatment of malignancies; however, no such effect has been shown in clinical trials. Hence, the causes underlying these conflicting results require further exploration. This review focuses on the clinical significance of glucose metabolism disorders in PDAC and the mechanisms behind this relationship, while also summarizing therapeutic approaches that target glycolysis.
Full article
►▼
Show Figures
Open AccessReview
Emerging Role of Targeted Therapy in Metastatic Pancreatic Adenocarcinoma
by
Brandon M. Huffman, Haley Ellis, Alexander C. Jordan, William A. Freed-Pastor, Kimberly Perez, Douglas A. Rubinson, Nilay Sethi, Harshabad Singh, Rishi Surana, Brian M. Wolpin, Andrew J. Aguirre and James M. Cleary
Cited by 17 | Viewed by 5133
Abstract
The aggressive biology of pancreatic ductal adenocarcinoma (PDAC), along with its limited sensitivity to many systemic therapies, presents a major challenge in the management of patients with metastatic PDAC. Over the past decade, the incorporation of combinatorial cytotoxic chemotherapy regimens has improved patient
[...] Read more.
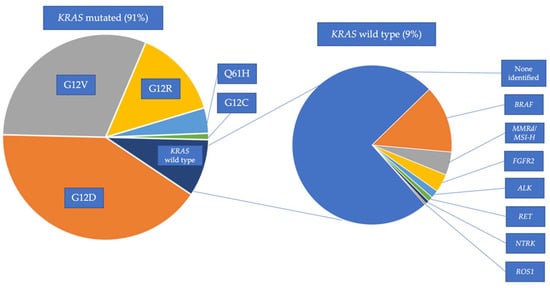
The aggressive biology of pancreatic ductal adenocarcinoma (PDAC), along with its limited sensitivity to many systemic therapies, presents a major challenge in the management of patients with metastatic PDAC. Over the past decade, the incorporation of combinatorial cytotoxic chemotherapy regimens has improved patient outcomes. Despite these advances, resistance to cytotoxic chemotherapy inevitably occurs, and there is a great need for effective therapies. A major focus of research has been to identify molecularly defined subpopulations of patients with PDAC who may benefit from targeted therapies that are matched to their molecular profile. Recent successes include the demonstration of the efficacy of maintenance PARP inhibition in PDAC tumors harboring deleterious
BRCA1,
BRCA2, and
PALB2 alterations. In addition, while therapeutic targeting of
KRAS was long thought to be infeasible, emerging data on the efficacy of
KRAS G12C inhibitors have increased optimism about next-generation
KRAS-directed therapies in PDAC. Meanwhile,
KRAS wild-type PDAC encompasses a unique molecular subpopulation of PDAC that is enriched for targetable genetic alterations, such as oncogenic
BRAF alterations, mismatch repair deficiency, and
FGFR2,
ALK,
NTRK,
ROS1,
NRG1, and
RET rearrangements. As more molecularly targeted therapies are developed, precision medicine has the potential to revolutionize the treatment of patients with metastatic PDAC.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
The Right Treatment Strategy for the Right Patient: A Biomarker-Driven Approach to Neoadjuvant vs. Surgery-First Management of Resectable and Borderline Resectable Pancreatic Cancer
by
Christopher B. Nahm, John Turchini, Sumit Sahni, Elizabeth Moon, Malinda Itchins, Jennifer Arena, Angela Chou, Emily K. Colvin, Viive M. Howell, Nick Pavlakis, Stephen Clarke, Jaswinder S. Samra, Anthony J. Gill and Anubhav Mittal
Cited by 7 | Viewed by 2639
Abstract
The genomic heterogeneity of pancreatic ductal adenocarcinoma (PDAC) is becoming increasingly appreciated. We aimed to evaluate the ability of a triple biomarker panel (S100A4, Ca-125, and mesothelin) to predict: (i) genetic PDAC subtypes; (ii) clinical phenotypes; and (iii) the optimal treatment strategy (neoadjuvant
[...] Read more.
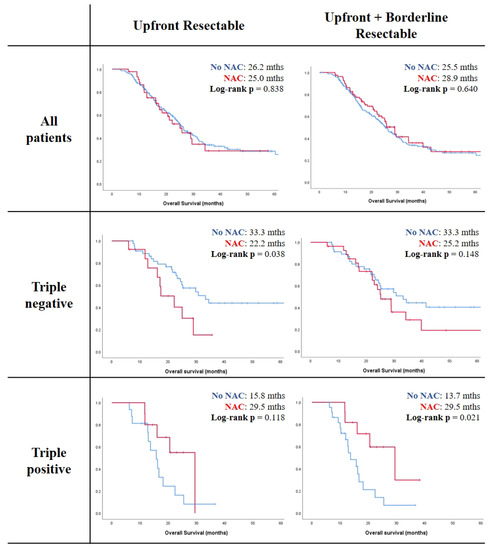
The genomic heterogeneity of pancreatic ductal adenocarcinoma (PDAC) is becoming increasingly appreciated. We aimed to evaluate the ability of a triple biomarker panel (S100A4, Ca-125, and mesothelin) to predict: (i) genetic PDAC subtypes; (ii) clinical phenotypes; and (iii) the optimal treatment strategy (neoadjuvant vs. surgery-first) in resectable and borderline resectable PDAC. Patients who underwent resection for resectable and borderline resectable PDAC were included from one single-institutional cohort and one multi-institutional cohort from the Australian Pancreatic Genome Initiative (APGI). Tumors were immunohistochemically evaluated for S100A4, Ca-125, and mesothelin, and a subset from the APGI cohort underwent RNA sequencing. This study included 252 and 226 patients from the single institution and the APGI cohorts, respectively. Triple-negative biomarker status correlated with non-squamous PDAC genotypes (
p = 0.020), lower rates of distant recurrence (
p = 0.002), and longer median overall survival (mOS) with the surgery-first approach compared with neoadjuvant treatment (33.3 vs. 22.2 mths,
p = 0.038) in resectable PDAC. In contrast, the triple-positive disease was associated with longer mOS with neoadjuvant treatment compared with the surgery-first approach (29.5 vs. 13.7 mths,
p = 0.021) in resectable and borderline resectable PDAC. In conclusion, the triple biomarker panel predicts genetic PDAC subtypes, clinical phenotypes, and optimal treatment strategies in resectable and borderline resectable PDAC.
Full article
►▼
Show Figures
Open AccessReview
Autophagy: A Key Player in Pancreatic Cancer Progression and a Potential Drug Target
by
Josef Gillson, Yomna S. Abd El-Aziz, Lionel Y. W. Leck, Patric J. Jansson, Nick Pavlakis, Jaswinder S. Samra, Anubhav Mittal and Sumit Sahni
Cited by 29 | Viewed by 6748
Abstract
Pancreatic cancer is known to have the lowest survival outcomes among all major cancers, and unfortunately, this has only been marginally improved over last four decades. The innate characteristics of pancreatic cancer include an aggressive and fast-growing nature from powerful driver mutations, a
[...] Read more.
Pancreatic cancer is known to have the lowest survival outcomes among all major cancers, and unfortunately, this has only been marginally improved over last four decades. The innate characteristics of pancreatic cancer include an aggressive and fast-growing nature from powerful driver mutations, a highly defensive tumor microenvironment and the upregulation of advantageous survival pathways such as autophagy. Autophagy involves targeted degradation of proteins and organelles to provide a secondary source of cellular supplies to maintain cell growth. Elevated autophagic activity in pancreatic cancer is recognized as a major survival pathway as it provides a plethora of support for tumors by supplying vital resources, maintaining tumour survival under the stressful microenvironment and promoting other pathways involved in tumour progression and metastasis. The combination of these features is unique to pancreatic cancer and present significant resistance to chemotherapeutic strategies, thus, indicating a need for further investigation into therapies targeting this crucial pathway. This review will outline the autophagy pathway and its regulation, in addition to the genetic landscape and tumor microenvironment that contribute to pancreatic cancer severity. Moreover, this review will also discuss the mechanisms of novel therapeutic strategies that inhibit autophagy and how they could be used to suppress tumor progression.
Full article
►▼
Show Figures