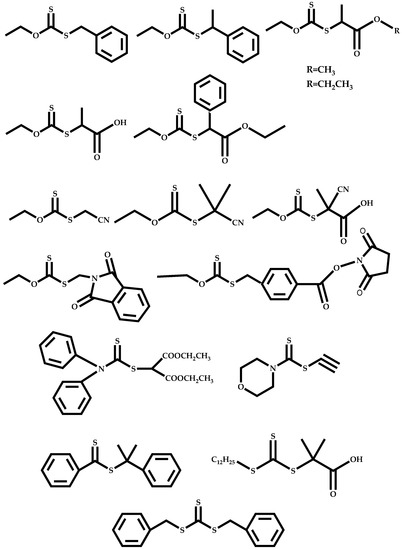
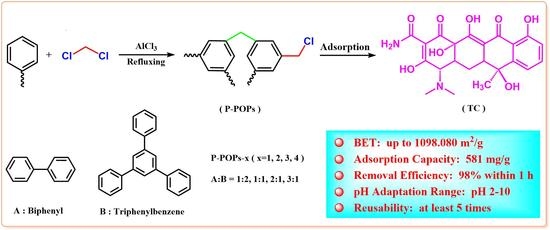
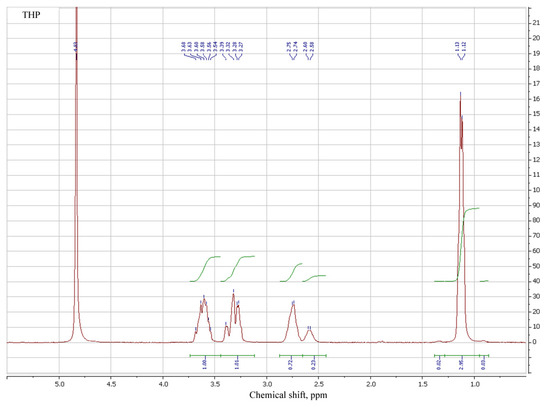
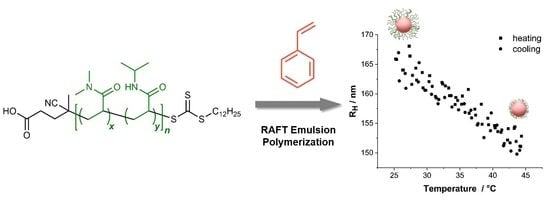
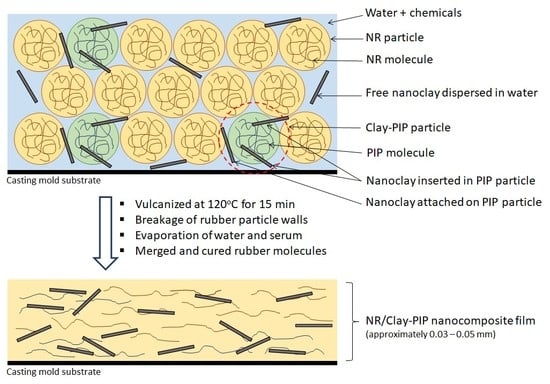
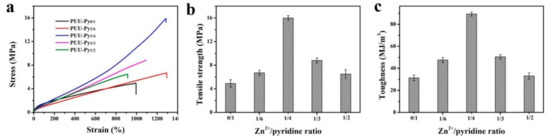
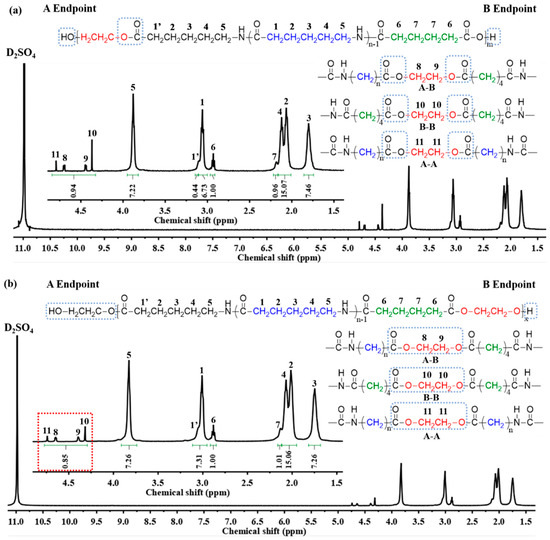
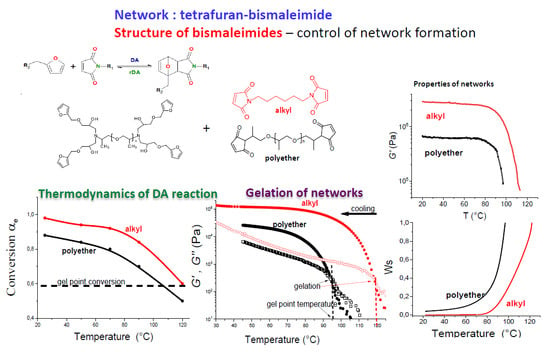
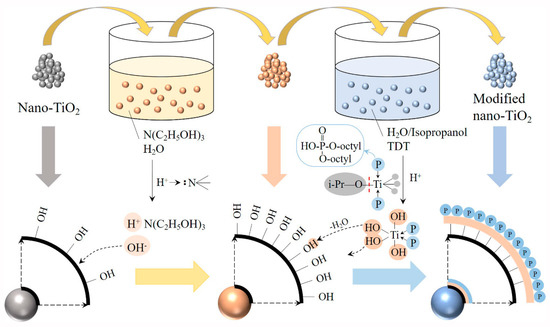
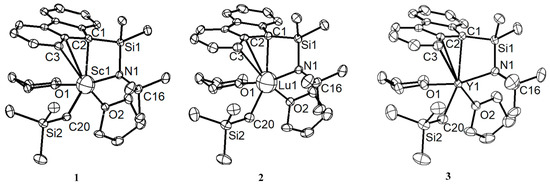
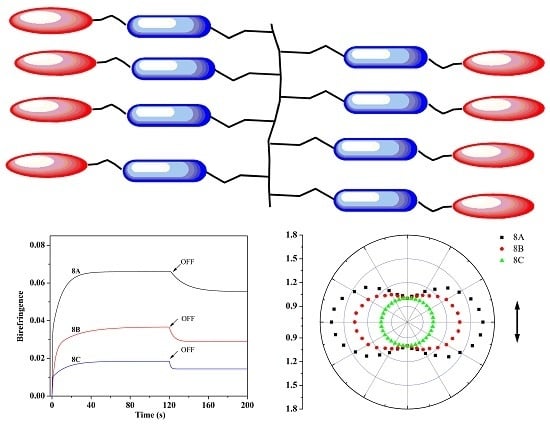
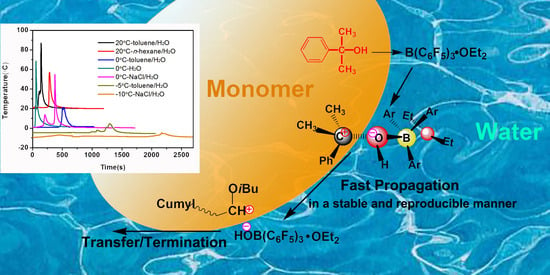
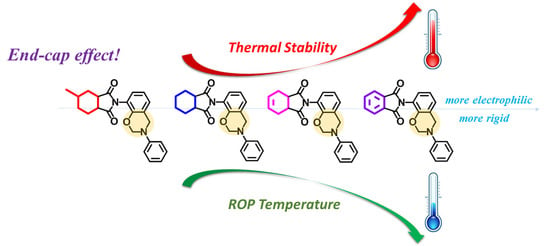
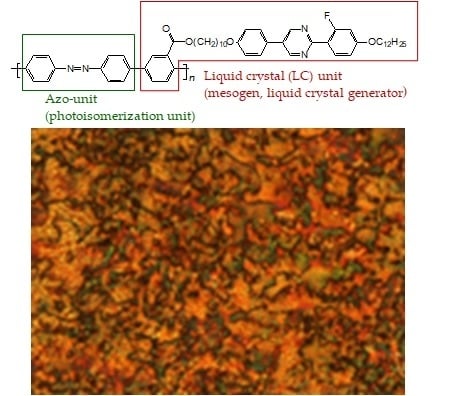
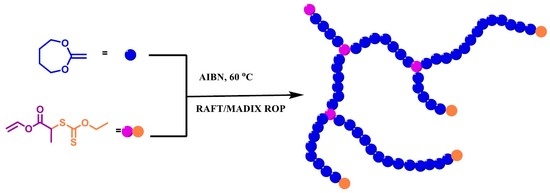
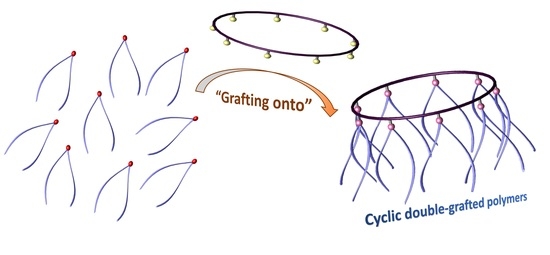
Design and Synthesis of Polymers
A topical collection in Polymers (ISSN 2073-4360). This collection belongs to the section "Polymer Chemistry".
Viewed by 187802Editor
Interests: controlled/living radical polymerization; RAFT; TERP; water-soluble polymer; self-organization; polymer micelle; bioconjugate polymer
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
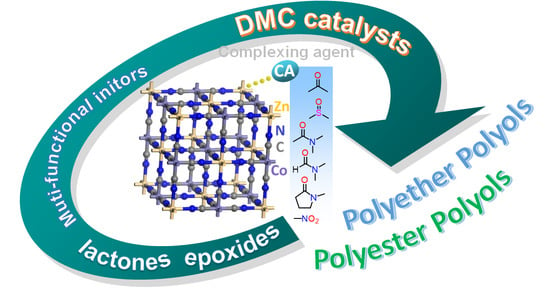
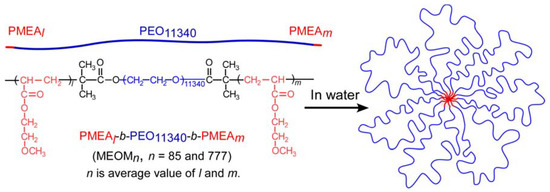
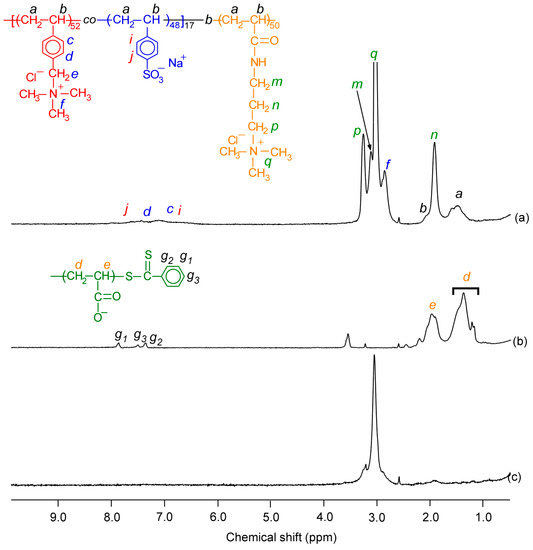
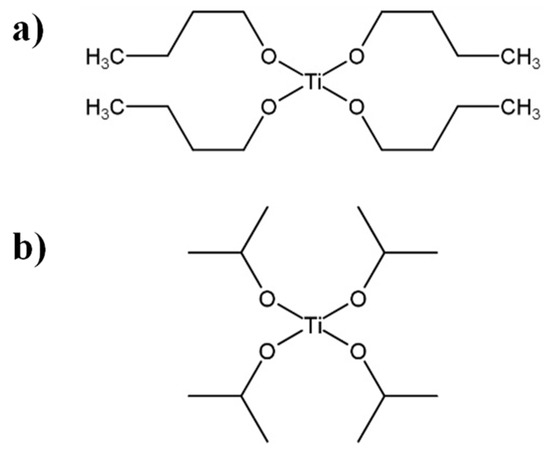
It is well-known that polymer functions strongly depend on their design and structure. The design and synthesis of polymers are very important for desired properties. Commonly, most polymers are synthesized by the following methods: radical, anionic, cationic, condensation, ring opening, and coordination polymerizations. Unfortunately, these synthetic polymer structures are not well-controlled compared with biopolymers, such as polypeptide, DNA, RNA, and so on. We cannot precisely control molecular weight, molecular weight distribution, monomer sequence, and tacticity. Recently, controlled polymerization methods have been developed to achieve well-controlled structured polymers with defined molecular weight and narrow molecular weight distribution. However, this is unsatisfactory compared with biopolymers. Especially, monomer sequence and tacticity controls constitute difficult challenges. Additionally, there are various design of polymers, such as linear polymer, random copolymer, alternative copolymer, block copolymer, branched polymer, graft copolymer, star-shape copolymer, mikto−arm copolymer, dendrimer, and so on. These designs are the key points of the functions.
This collection is concerned with the design and synthesis of polymers. We hope to share new concept designs of polymers, polymer synthesis methods, and analysis of polymerization mechanisms. Both original contributions and reviews are welcome.
Prof. Shin-ichi Yusa
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Polymers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Linear polymer
- Random copolymer
- Alternative copolymer
- Branched polymer
- Block copolymer
- Conjugated polymer
- Graft copolymer
- Star-shaped polymer
- Dendrimer
- Radical polymerization
- Anionic polymerization
- Cationic polymerization
- Condensation polymerization
- Ring opening polymerization
- Coordination polymerization
- Polyaddition
- Addition condensation
- Living polymerization
- Polymerization mechanism
- Monomer sequence control
- Tactility
- Copolymerization
- Photo-polymerization
- New concept of polymerization