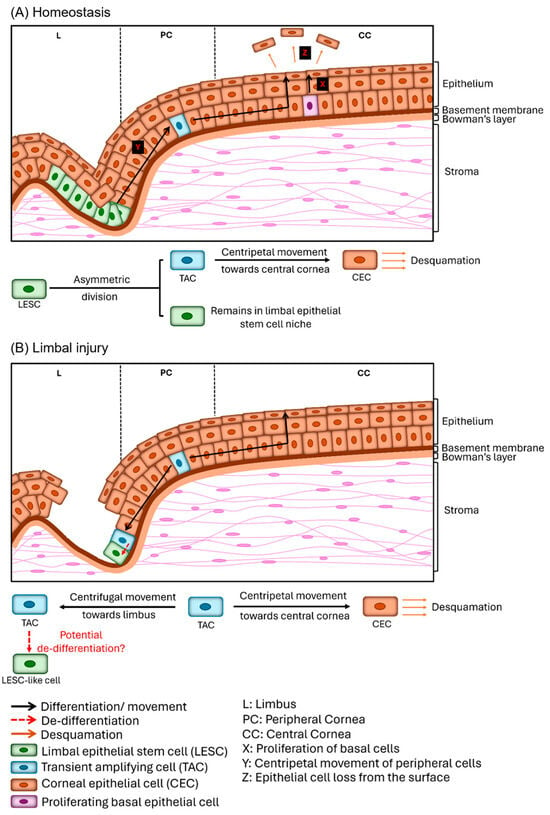
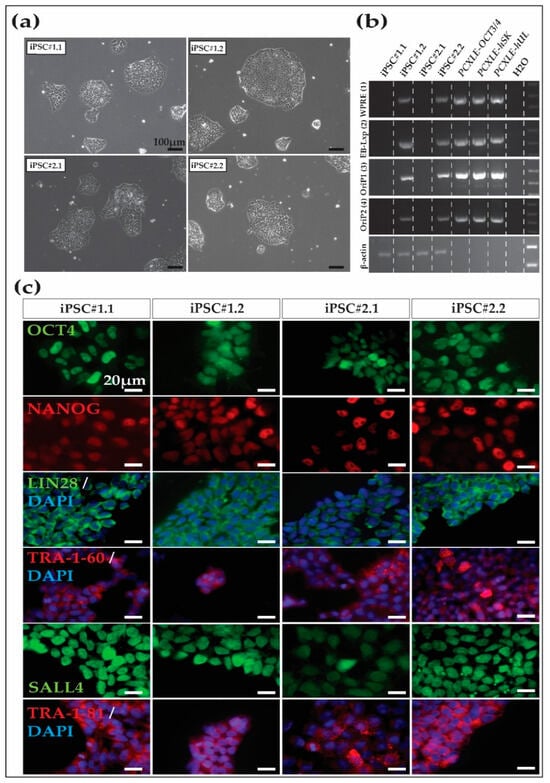
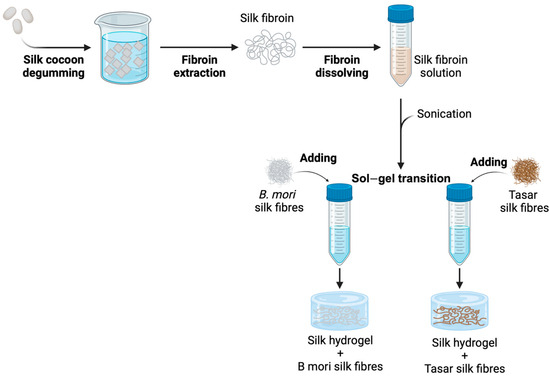
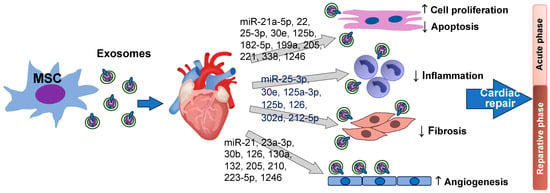
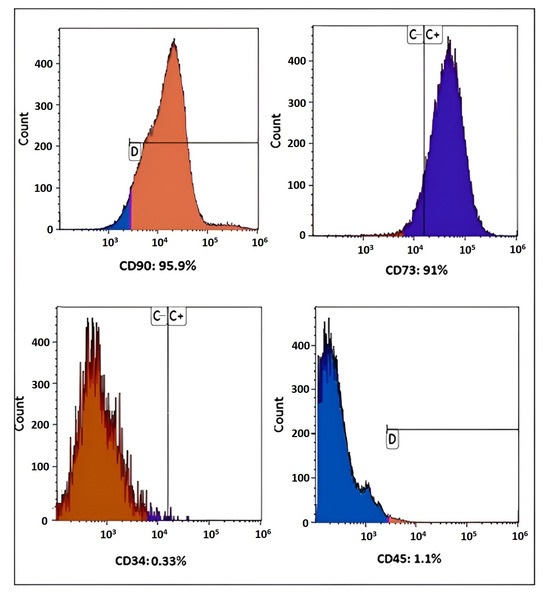
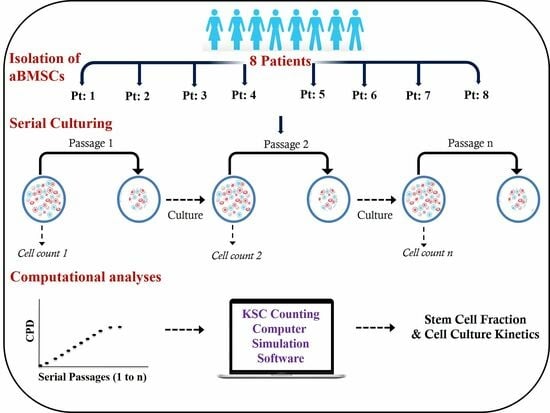
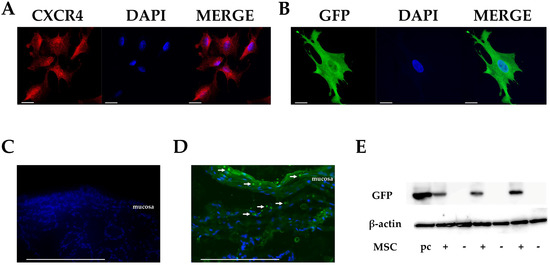
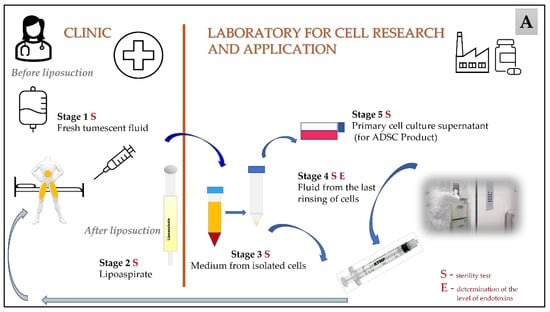
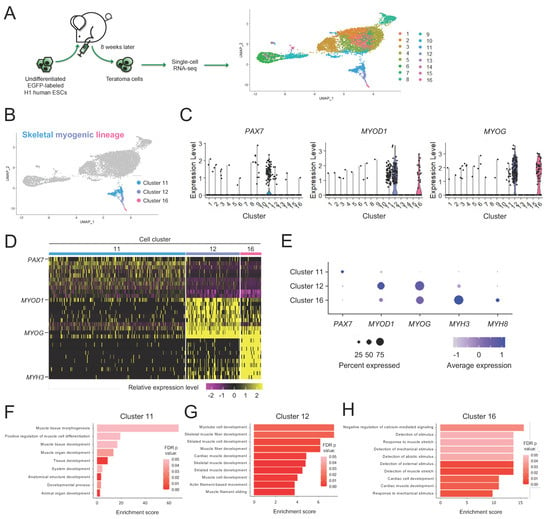
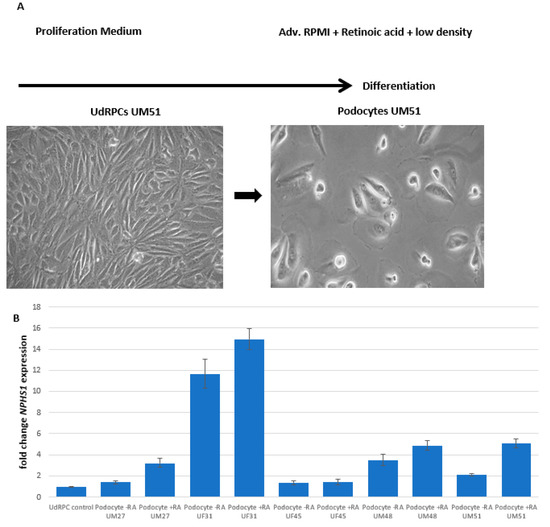
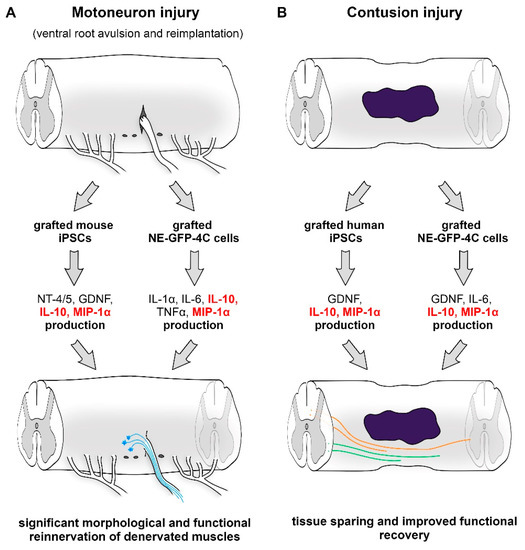
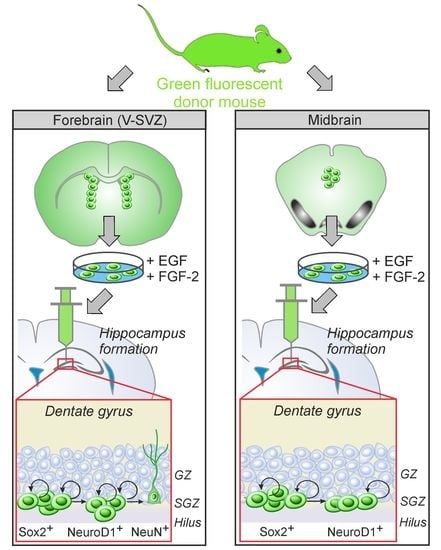
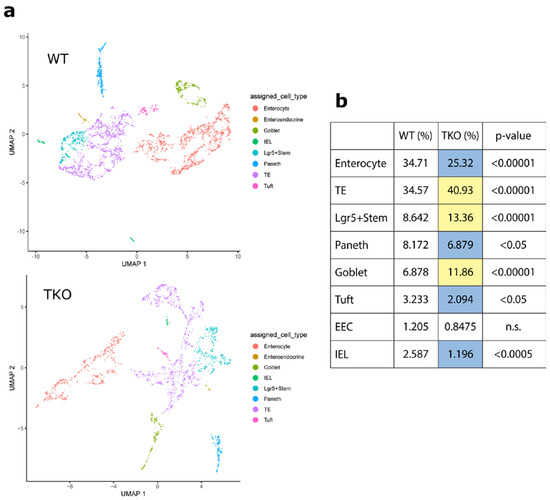
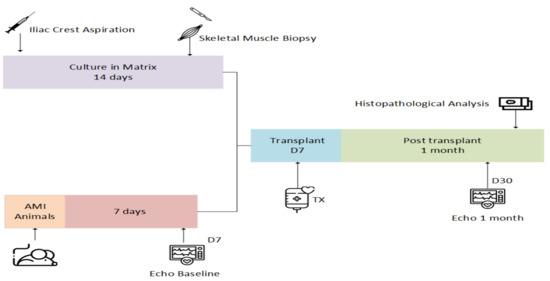
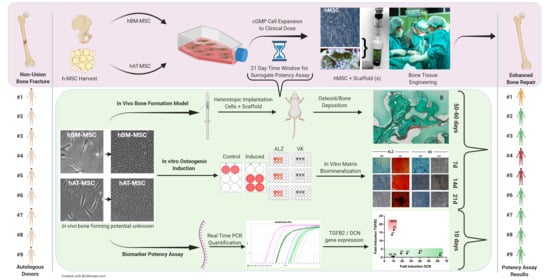
Stem Cells in Tissue Engineering and Regeneration
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 128555Editor
Interests: stem cell biology; extracellular vesicle (EV) biology; medical biotechnology; regenerative medicine; experimental cardiology (including clinical studies).
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
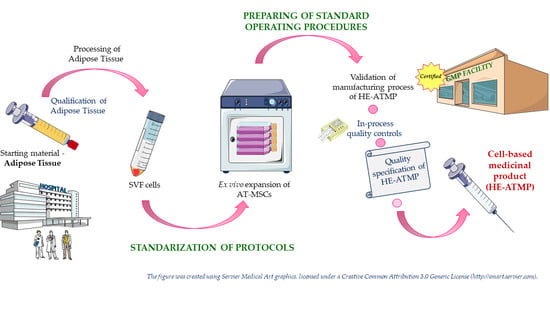
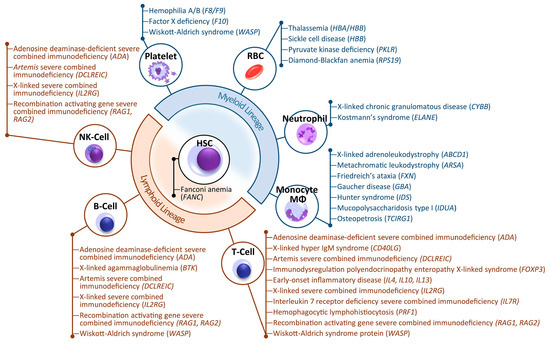
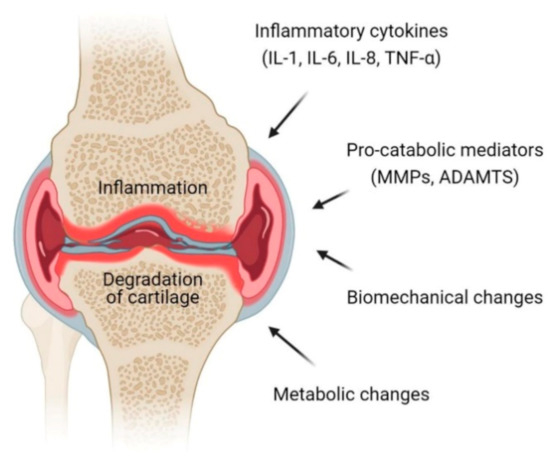
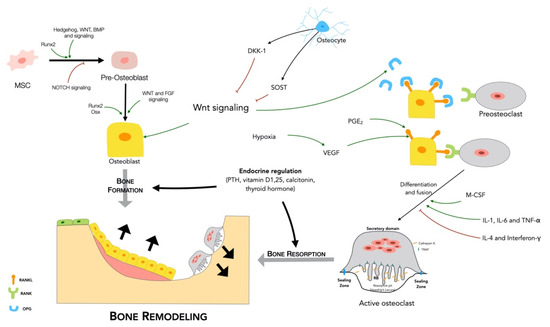
Stem cells (SCs) of various origin and types have been the subject of several worldwide studies and clinical applications targeting several human diseases and tissue injuries over the last several years. Although multiple therapeutic approaches have already been proposed based on identified SC biological properties and mechanisms of action, there remains a need for: (i) a better understanding of the processes underlying the pro-reparative activity of various SC populations in various tissues; (ii) their targeted use in various tissue injuries and patients; and (iii) an enhancement of the pro-regenerative capacity of these cells prior to transplantation in vivo. Thus, several novel approaches to SC-based tissue engineering and regeneration are currently being developed in order to take stem cell biology and SC use in tissue repair to the ‘4th generation of cell-based therapies’, such as: (i) ‘cell-free’ approaches relying on the secretomic activity of SCs, including extracellular vesicle (EV) release; (ii) combining SCs with novel biocompatible scaffolds and artificial sheets to enhance their properties and form innovative tissue/organ replacements; (iii) three-dimensional (3D) ex vivo propagation and pre-treatment of SCs in conditions mimicking a specific niche of a certain tissue; (iv) genetic modifications of SCs leading to the pro-reparative properties required for repair of a certain tissue; and (v) development of GMP protocols for standardized ex vivo preparation of advanced SC-based therapies and medicinal products for use in human patients. This Topical Collection aims to present pioneering research and innovative approaches in the fields of stem cell biology, tissue engineering, and regenerative medicine.
I would like to warmly invite you to submit your unpublished original research as well as reviews that provide a comprehensive overview of a selected topic related to the fascinating subject of this Topical Collection of Cells.
Prof. Dr. Ewa Zuba-Surma
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- stem cell therapy
- mechanisms of tissue repair
- paracrine activity
- extracellular vesicles
- stem cell differentiation
- adult stem cells
- pluripotent stem cells
- tissue engineering
- biomaterials
- gene modification
- preclinical in vivo models
- GMP and advanced cell therapy medicinal products
Planned Papers
The below list represents only planned manuscripts. Some of these manuscripts have not been received by the Editorial Office yet. Papers submitted to MDPI journals are subject to peer-review.
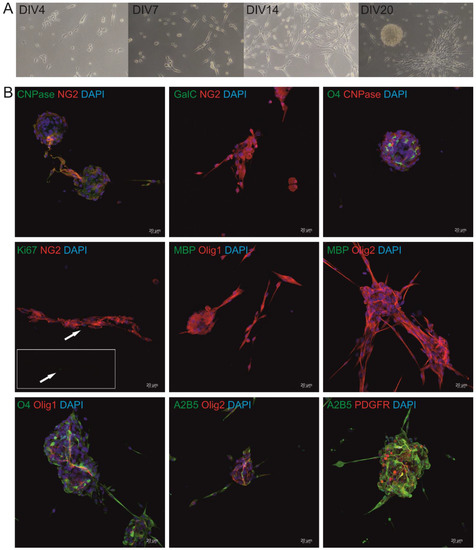
Title: Heterogeneity of glial cells is ideal therapeutic targets for future clinical strategies
Authors: Vyacheslav Dyachuk; et al.
Affiliation: Karolinska Institutetdisabled, Stockholm, Sweden
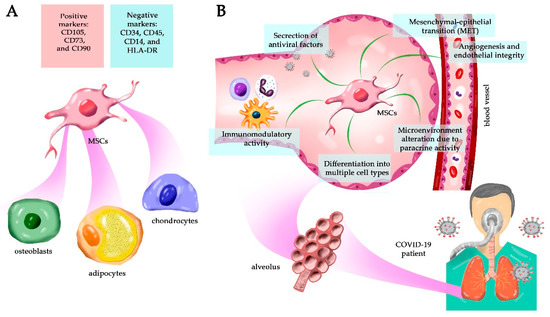
Title: Secretome from mesenchymal stem cells as therapy to treat age-related diseases
Authors: Juan Antonio Fafián-Labora; et al.
Affiliation: Queen Mary University of London, London, UK
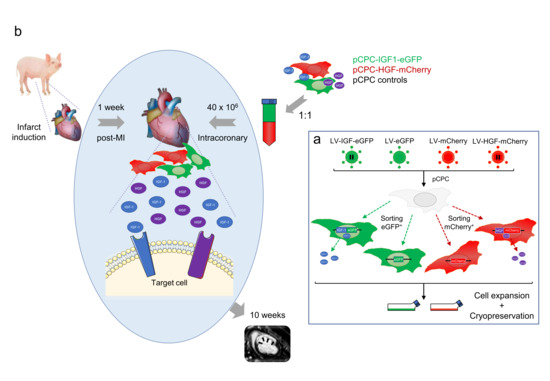
Title: In situ Activation of Endogenous Stem Cells: Ultrasound-mediated Drug Delivery for Tissue Regeneration
Authors: Gadi Pelled; Dan Gazit; et al.
Affiliation: Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA