Corrosion Behavior of Pre-Rusted Rebars in Cement Mortar Exposed to Harsh Environments
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
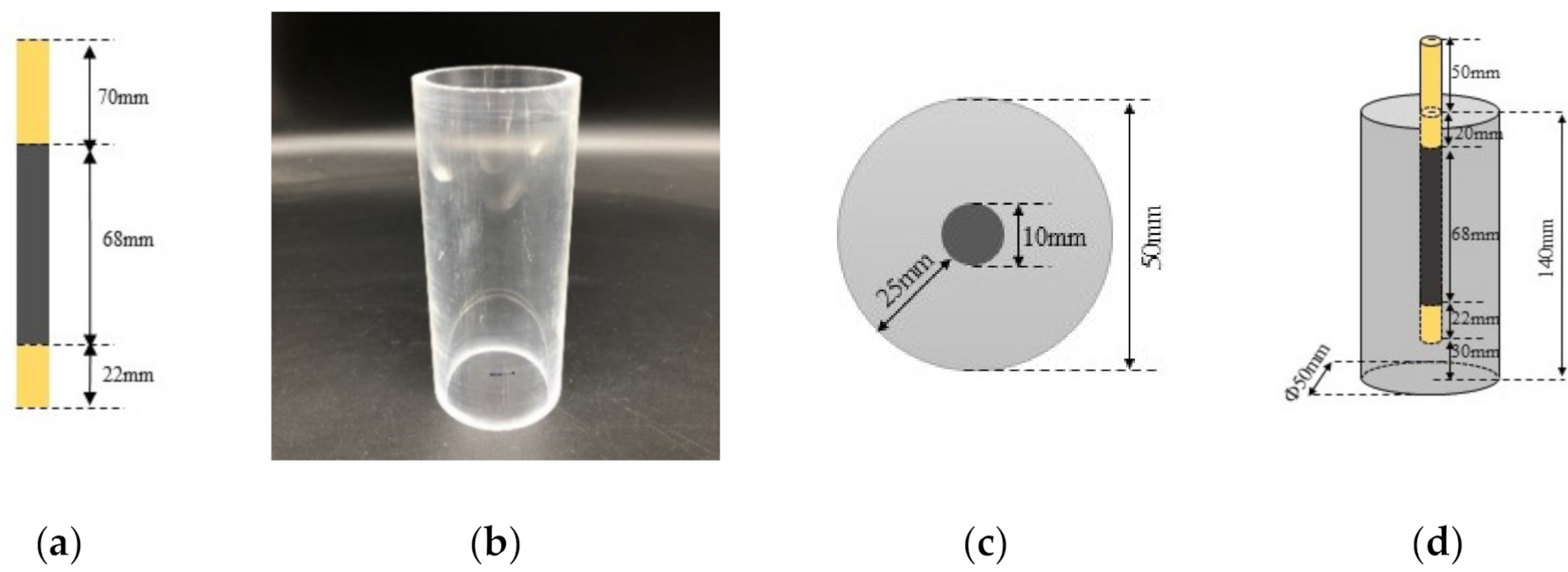
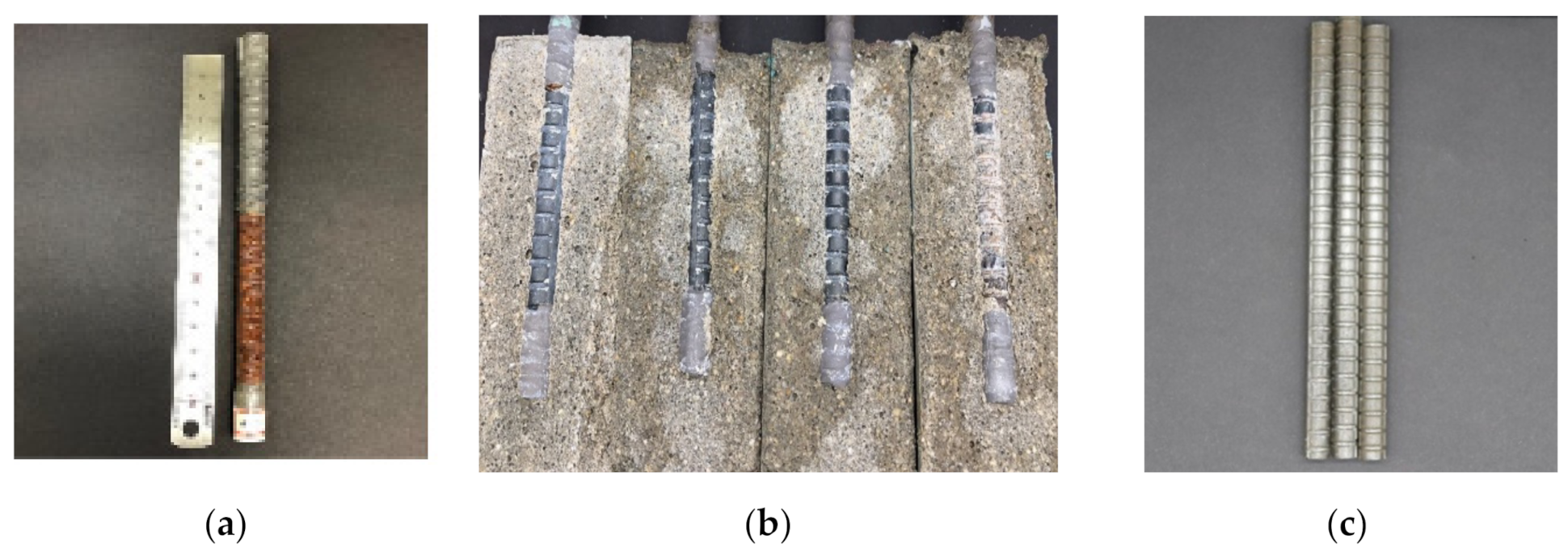
2.2. Specimen Preparation
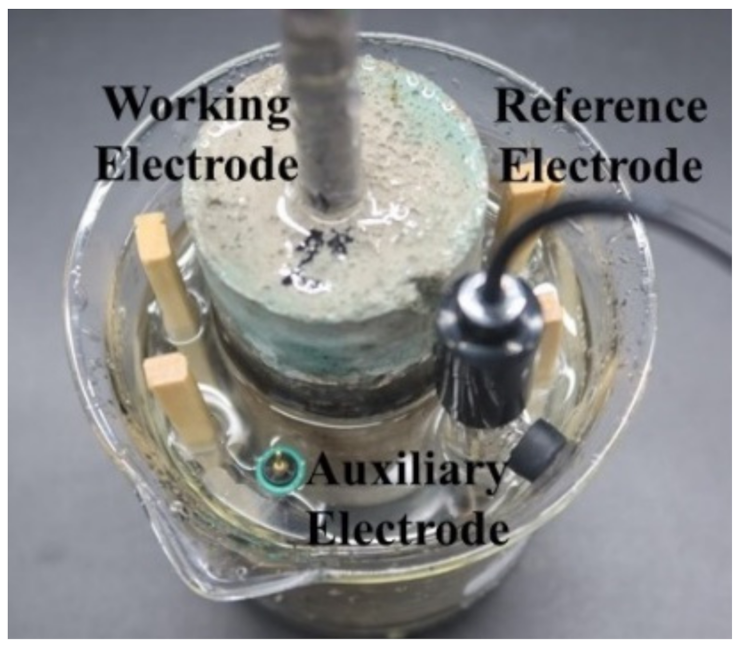
2.3. Measurement Method
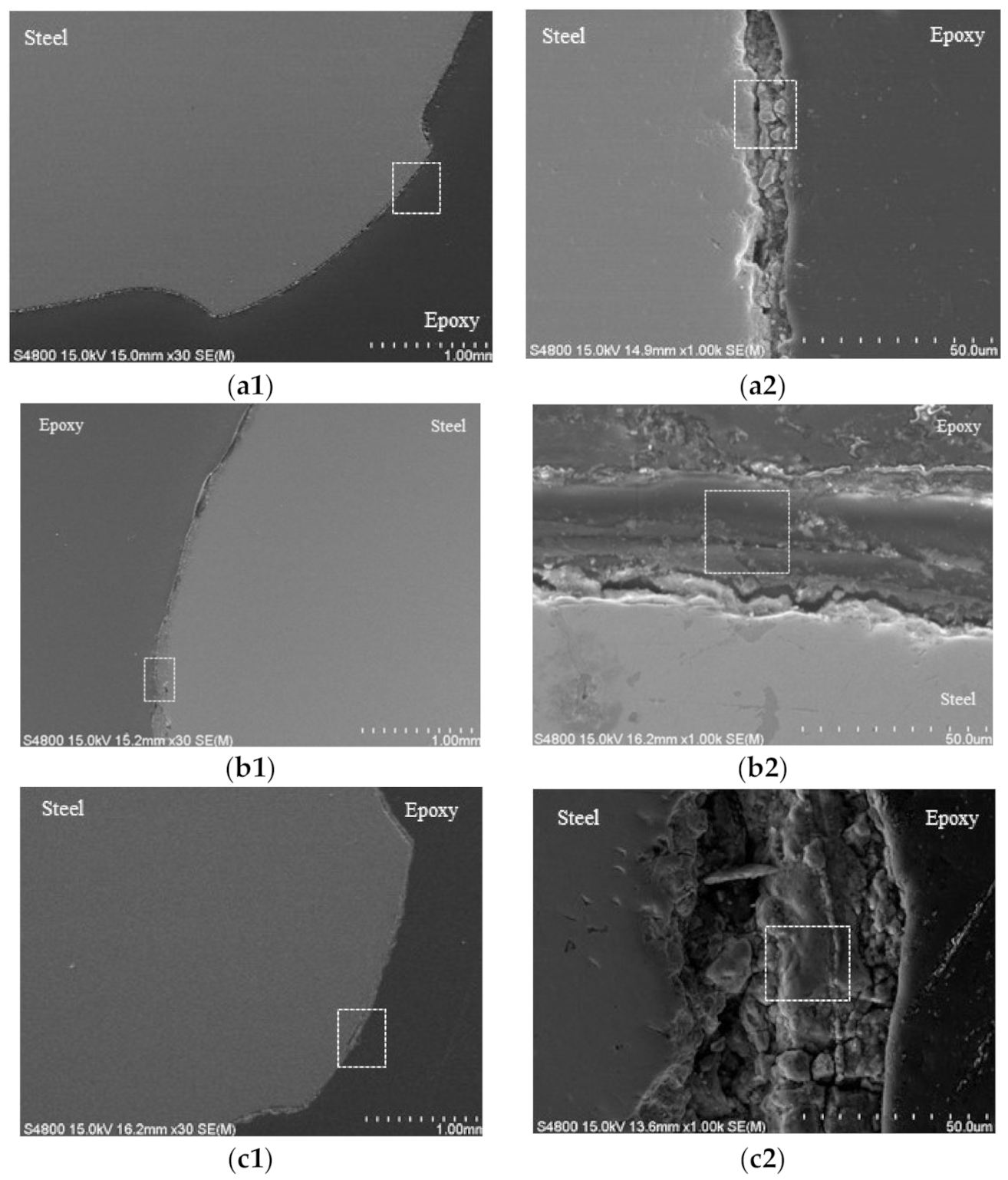
2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectrometer (EDX) Analysis
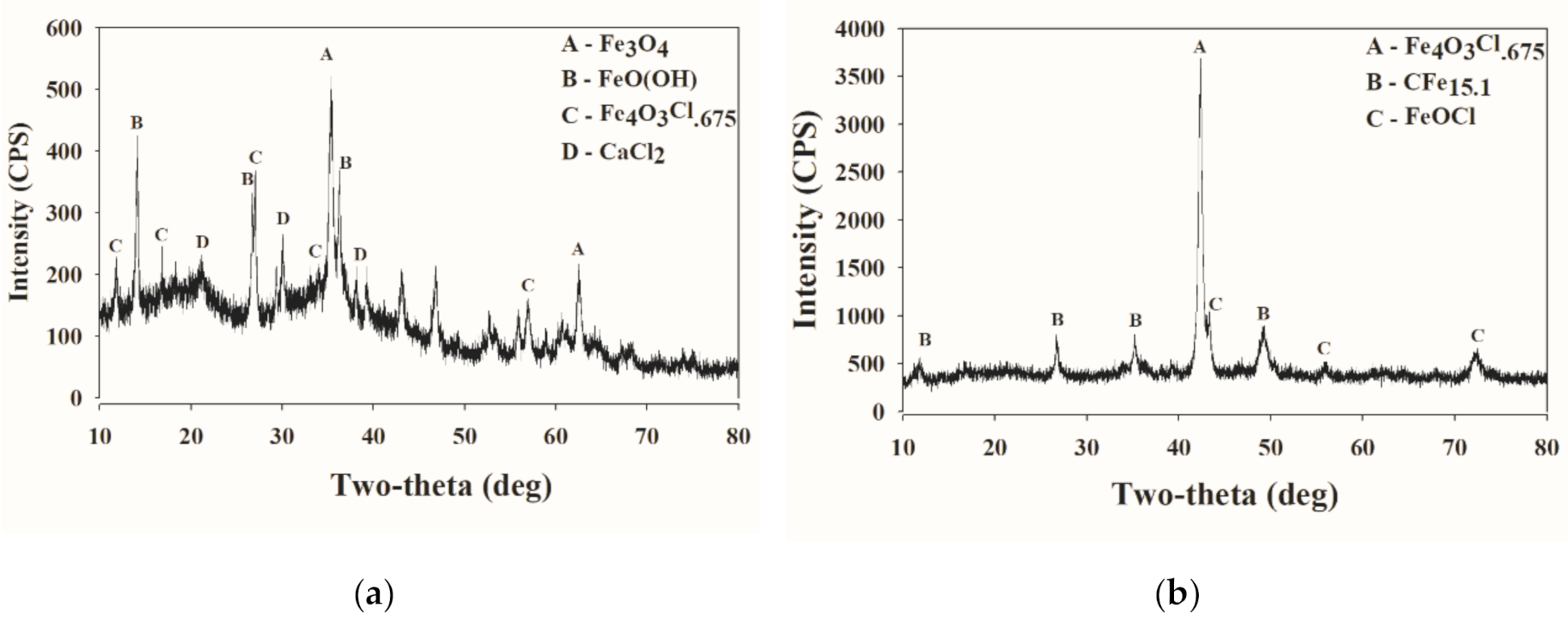
2.5. X-ray Diffraction Spectrometer (XRD) Measurement
3. Results
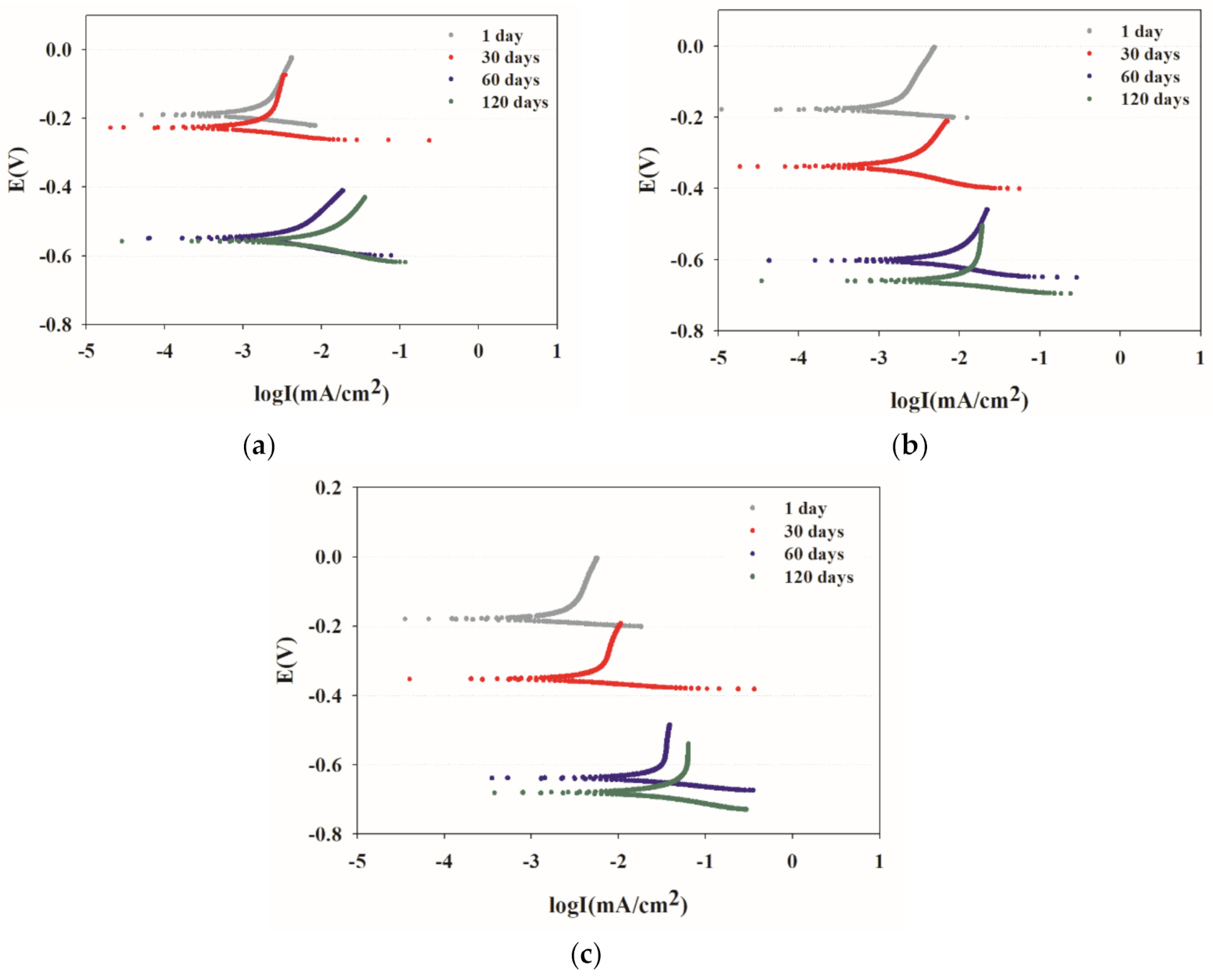
3.1. Electrochemical Measurements
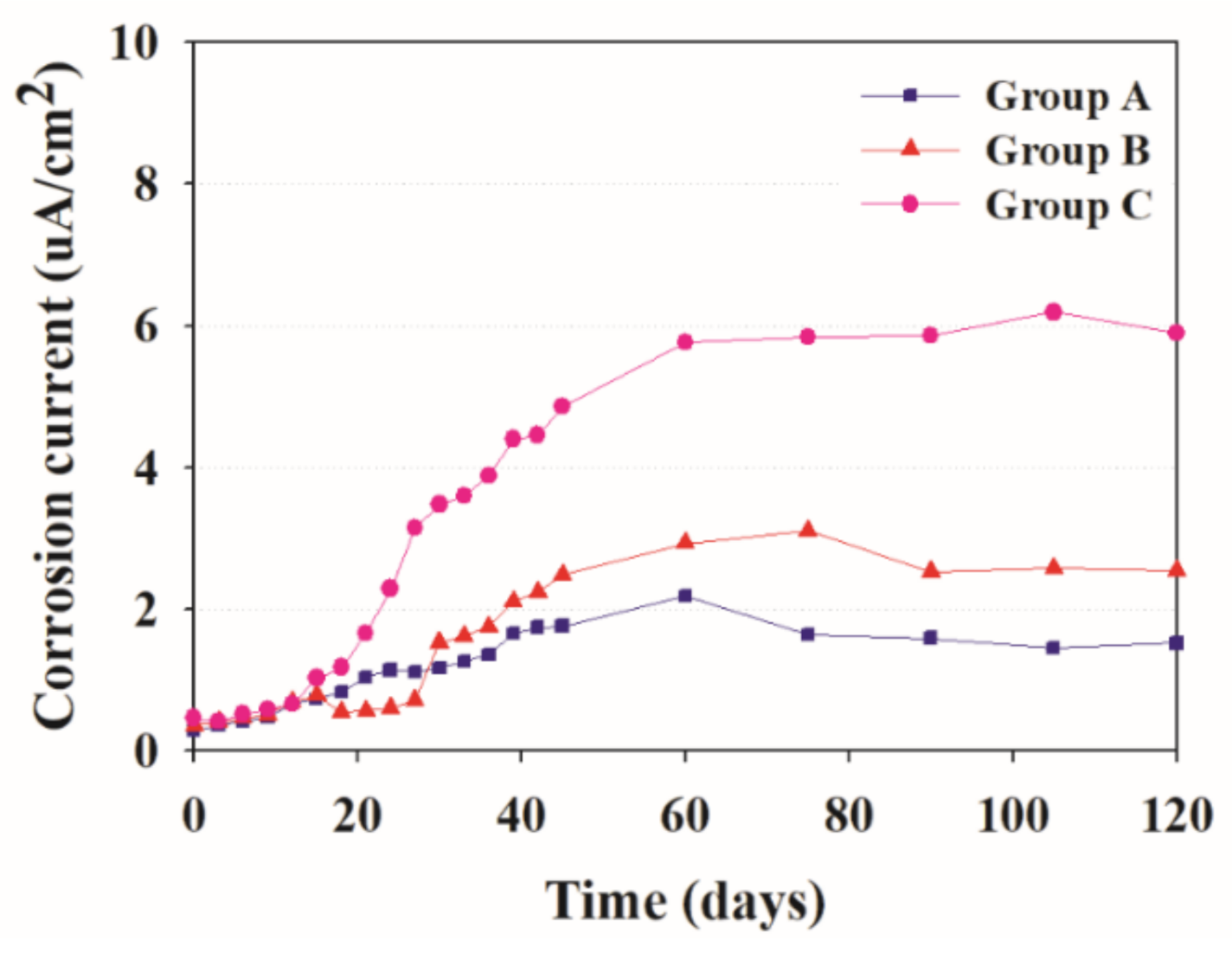
3.2. Corrosion Current
3.3. Half-Cell Potential
3.4. Scanning Electron Microscopy Analysis and X-ray Diffraction
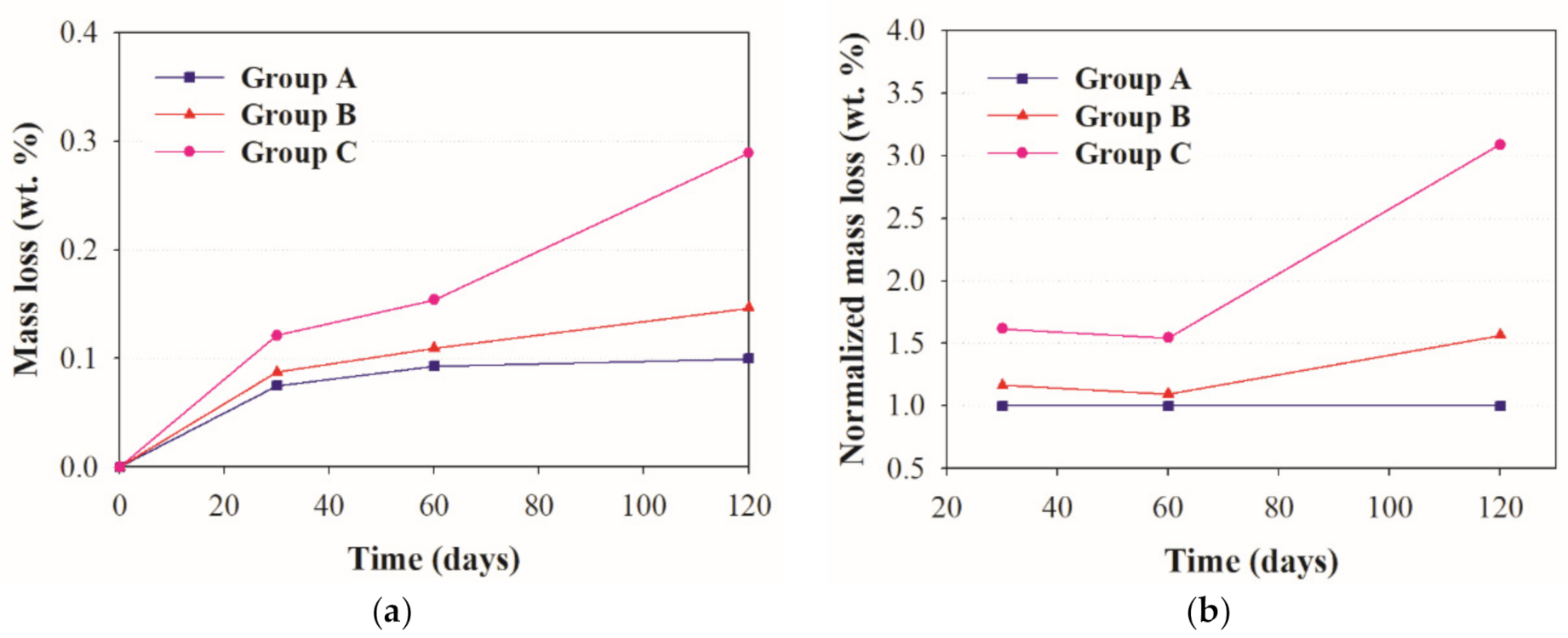
3.5. Visual Observation and Mass Loss
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uthaman, S.; George, R.P.; Vishwakarma, V.; Harilal, M.; Philip, J. Enhanced seawater corrosion resistance of reinforcement in nanophase modified fly ash concrete. Constr. Build. Mater. 2019, 221, 232–243. [Google Scholar] [CrossRef]
- Revie, R.W. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C.; et al. The effect of the steel–concrete interface on chloride-induced corrosion initiation in concrete: A critical review by RILEM TC 262-SCI. Mater. Struct. 2019, 52, 88. [Google Scholar] [CrossRef]
- Angst, U.M. Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. 2018, 51, 4. [Google Scholar] [CrossRef] [Green Version]
- Kumar Sharma, D.; Filipponi, M.; Di Schino, A.; Rossi, F.; Castaldi, J. Corrosion behaviour of high temperature fuel cells: Issues for materials selection. Metalurgija 2019, 58, 347–351. [Google Scholar]
- Di Schino, A. Manufacturing and Applications of Stainless Steels. Metals 2020, 10, 327. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, T.U.; Hamada, H. Corrosion of steel bars in concrete with various steel surface conditions. ACI Mater. J. 2006, 103, 233–242. [Google Scholar]
- Bensabra, H.; Azzouz, N. Study of rust effect on the corrosion behavior of reinforcement steel using impedance spectroscopy. Metall. Mater. Trans. A 2013, 44, 5703–5710. [Google Scholar] [CrossRef]
- Ismail, A.I.M.; El-Shamy, A.M. Engineering behaviour of soil materials on the corrosion of mild steel. Appl. Clay Sci. 2009, 42, 356–362. [Google Scholar] [CrossRef]
- Zhang, J.; Hosemann, P.; Maloy, S. Models of liquid metal corrosion. J. Nucl. Mater. 2010, 404, 82–96. [Google Scholar] [CrossRef]
- Shalon, R.; Rapheal, M. Influence of sea water on corrosion of reinforcement. J. Proc. 1959, 55, 1251–1268. [Google Scholar]
- Martinez-Echevarria, M.J.; Lopez-Alonso, M.; Romero, D.C.; Montero, J.R. Influence of the previous state of corrosion of rebars in predicting the service life of reinforced concrete structures. Constr. Build. Mater. 2018, 188, 915–923. [Google Scholar] [CrossRef]
- Fang, C.; Lundgren, K.; Chen, L.; Zhu, C. Corrosion influence on bond in reinforced concrete. Cem. Concr. Res. 2004, 34, 2159–2167. [Google Scholar] [CrossRef]
- Chung, L.; Kim, J.-H.J.; Yi, S.-T. Bond strength prediction for reinforced concrete members with highly corroded reinforcing bars. Cem. Concr. Compos. 2008, 30, 603–611. [Google Scholar] [CrossRef]
- Abosrra, L.; Ashour, A.F.; Youseffi, M. Corrosion of steel reinforcement in concrete of different compressive strengths. Constr. Build. Mater. 2011, 25, 3915–3925. [Google Scholar] [CrossRef]
- Kielė, A.; Vaitkevičius, V.; Sasnauskas, V.; Vaičiukyniene, D.; Bistrickaitė, R. Reinforcement Corrosion Degree Effect on Adhesion with Concrete. J. Sustain. Arch. Civ. Eng. 2014, 7. [Google Scholar] [CrossRef]
- Pradhan, B. Corrosion behavior of steel reinforcement in concrete exposed to composite chloride–sulfate environment. Constr. Build. Mater. 2014, 72, 398–410. [Google Scholar] [CrossRef]
- Oh, K.-S.; Moon, J.-M.; Park, K.-T.; Kwon, S.-J. Evaluation of Load Capacity Reduction in RC Beam with Corroded FRP Hybrid Bar and Steel. J. Korea Inst. Struct. Maint. Insp. 2016, 20, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gong, J.; Wang, L. Seismic behavior of corrosion-damaged reinforced concrete columns strengthened using combined carbon fiber-reinforced polymer and steel jacket. Constr. Build. Mater. 2009, 23, 2653–2663. [Google Scholar] [CrossRef]
- Novak, P.; Mala, R.; Joska, L. Influence of pre-rusting on steel corrosion in concrete. ACI J. Proc. 2001, 31, 589–593. [Google Scholar] [CrossRef]
- Al-Tayyib, A.; Khan, M.; Allam, I.; Al-Mana, A. Corrosion behavior of pre-rusted rebars after placement in concrete. Cem. Concr. Res. 1990, 20, 955–960. [Google Scholar] [CrossRef]
- Gonzalez, J.; Ramírez, E.; Bautista, A.; Feliu, S. The behaviour of pre-rusted steel in concrete. Cem. Concr. Res. 1996, 26, 501–511. [Google Scholar] [CrossRef]
- Kim, J.-S.; Shin, Y.-S.; Lee, S.; Kim, K.-S. The effect of chloride on corrosion of reinforced concrete. J. Korea Inst. Struct. Maint. Insp. 2005, 9, 2. [Google Scholar]
- Poursaee, A.; Hansson, C.M. Reinforcing steel passivation in mortar and pore solution. Cem. Concr. Res. 2007, 37, 1127–1133. [Google Scholar] [CrossRef]
- Beom, W.-J.; Yun, K.-S.; Park, C.-J.; Ryu, H.-J.; Kim, Y.-H. Comparison of influences of NaCl and CaCl2 on the corrosion of 11% and 17% Cr ferritic stainless steels during cyclic corrosion test. Corros. Sci. 2010, 52, 734–739. [Google Scholar] [CrossRef]
- Maslehuddin, M.; Al-Zahrani, M.; Al-Dulaijan, S.; Abdulquddus; Rehman, S.; Ahsan, S. Effect of steel manufacturing process and atmospheric corrosion on the corrosion-resistance of steel bars in concrete. Cem. Concr. Compos. 2002, 24, 151–158. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; McRae, G.; Gu, G.P.; Li, J. Effect of surface condition on the chloride-induced depassivation of rebar in concrete. In Proceedings of the 12th International Conference on Fracture (ICF), Ottawa, Canada CD Proceeding, Ottawa, ON, Canada, 12 July 2009. [Google Scholar]
- Doi, K.; Hiromoto, S.; Shinohara, T.; Tsuchiya, K.; Katayama, H.; Akiyama, E. Role of mill scale on corrosion behavior of steel rebars in mortar. Corros. Sci. 2020, 177, 108995. [Google Scholar] [CrossRef]
- Geng, J.; Liu, J.; Yan, J.; Ba, M.; He, Z.; Li, Y. Chemical composition of corrosion products of rebar caused by carbonation and chloride. Int. J. Corros. 2018, 2018, 7479383. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.S.; Megahed, H.E.; Fouad, N.; Elbahrawi, N.M. Corrosion inhibition of carbon steel in 1 M hydrochloric acid solution by aqueous extract of Thevetia peruviana. J. Bio-Tribo-Corros. 2016, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, D.; Díaz, I.; Alcántara, J.; Chico, B.; Simancas, J.; Llorente, I.; García-Delgado, A.; Jiménez, J.A.; Adeva, P.; Morcillo, M. Corrosion mechanisms of mild steel in chloride-rich atmospheres. Mater. Corros. 2016, 67, 227–238. [Google Scholar] [CrossRef]

| C | Si | Mn | P | S | Cu | N | C Eq 1 | |
|---|---|---|---|---|---|---|---|---|
| SD400 | 0.27 | 0.13 | 0.49 | 0.014 | 0.013 | 0.33 | 0.01 | 0.40 |
| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | SO3 | K2O | Na2O | TiO3 | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|
| 18.2 | 4.87 | 70.5 | 1.32 | 3.32 | 0.27 | 0.53 | 0.02 | 0.32 | 0.65 |
| Cement (g) | Fine Aggregate (g) | Water (mL) |
|---|---|---|
| 1500 | 4125 | 726 |
| Specimen | S | P | Mn | O | Fe | C | Cl | Ca |
|---|---|---|---|---|---|---|---|---|
| A | - | - | - | - | 97.52 | 2.48 | - | - |
| B | 2.31 | 0.3 | 0.7 | 32.74 | 62.01 | - | 1.55 | 0.4 |
| C | - | 1.14 | 0.74 | 20.49 | 77.45 | - | 0.18 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burtuujin, G.; Son, D.; Jang, I.; Yi, C.; Lee, H. Corrosion Behavior of Pre-Rusted Rebars in Cement Mortar Exposed to Harsh Environments. Appl. Sci. 2020, 10, 8705. https://doi.org/10.3390/app10238705
Burtuujin G, Son D, Jang I, Yi C, Lee H. Corrosion Behavior of Pre-Rusted Rebars in Cement Mortar Exposed to Harsh Environments. Applied Sciences. 2020; 10(23):8705. https://doi.org/10.3390/app10238705
Chicago/Turabian StyleBurtuujin, Gankhuyag, Dasom Son, Indong Jang, Chongku Yi, and Hyerin Lee. 2020. "Corrosion Behavior of Pre-Rusted Rebars in Cement Mortar Exposed to Harsh Environments" Applied Sciences 10, no. 23: 8705. https://doi.org/10.3390/app10238705





