Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato
Abstract
:1. Introduction
2. Materials and the Methods
2.1. Plasmid Construction and Tomato Transformation
2.2. Plant Materials and Growth Conditions
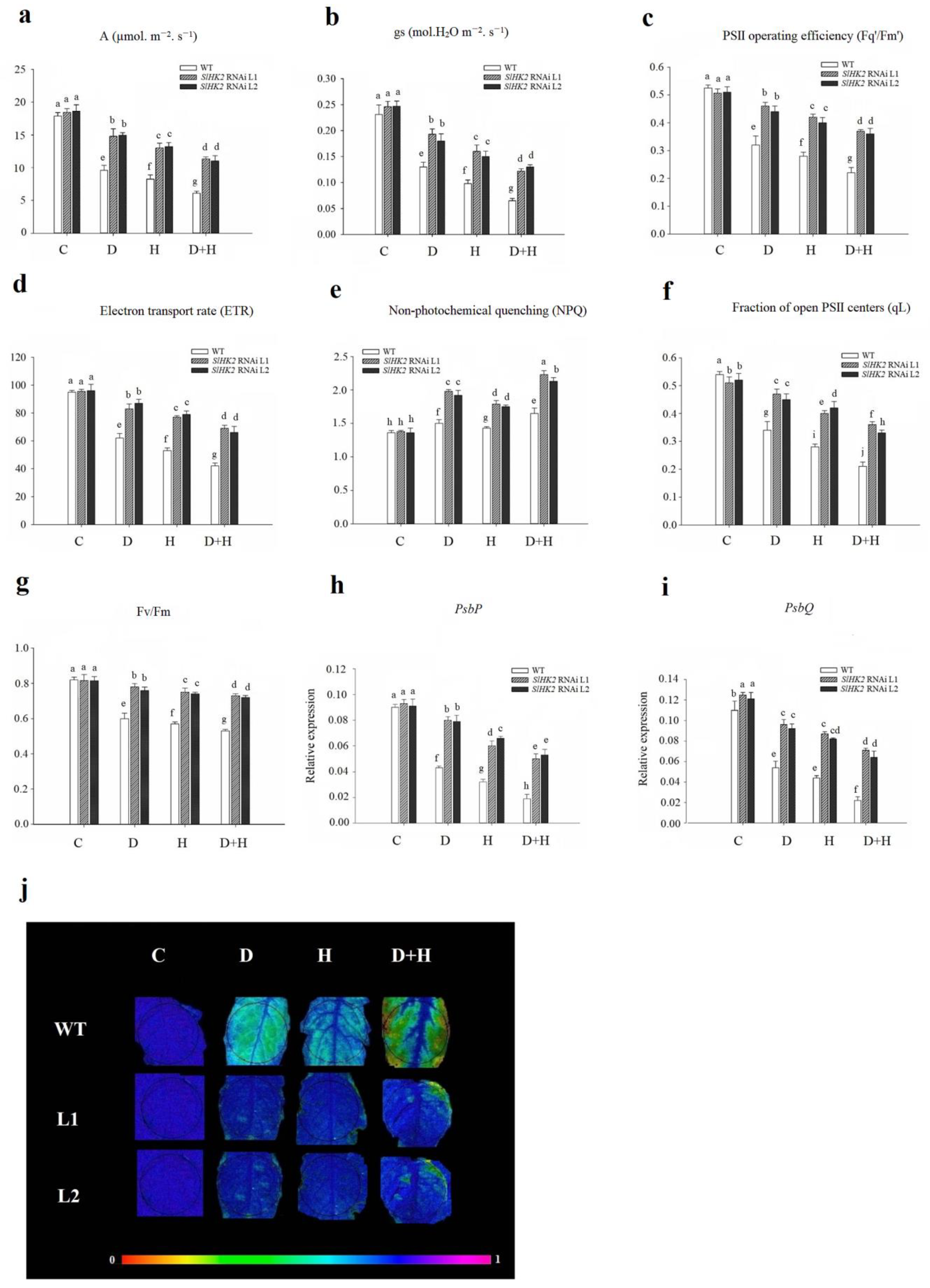
2.3. Gas Exchange and Chlorophyll Fluorescence Parameters
2.4. Determination of Osmoprotectant Accumulation
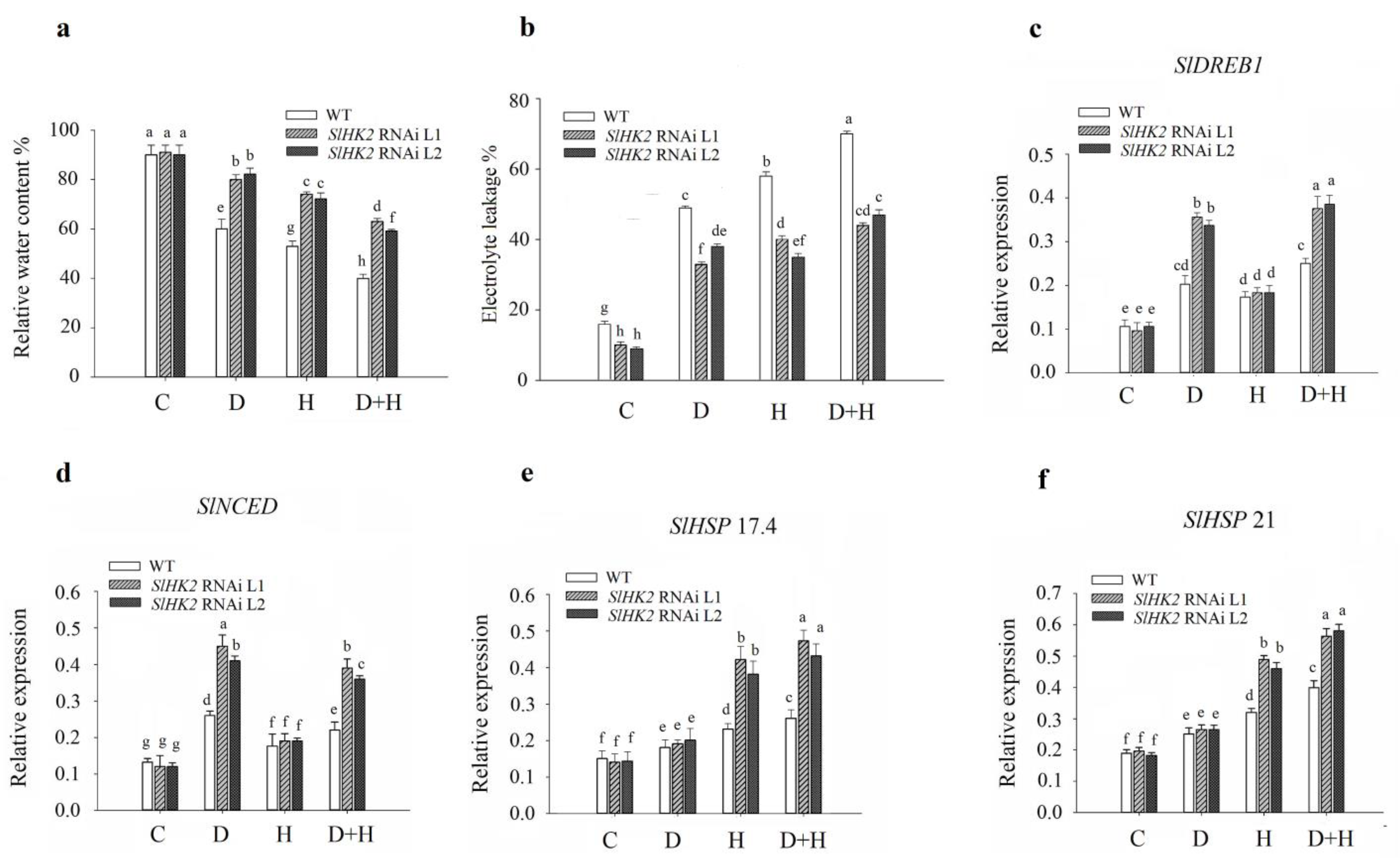
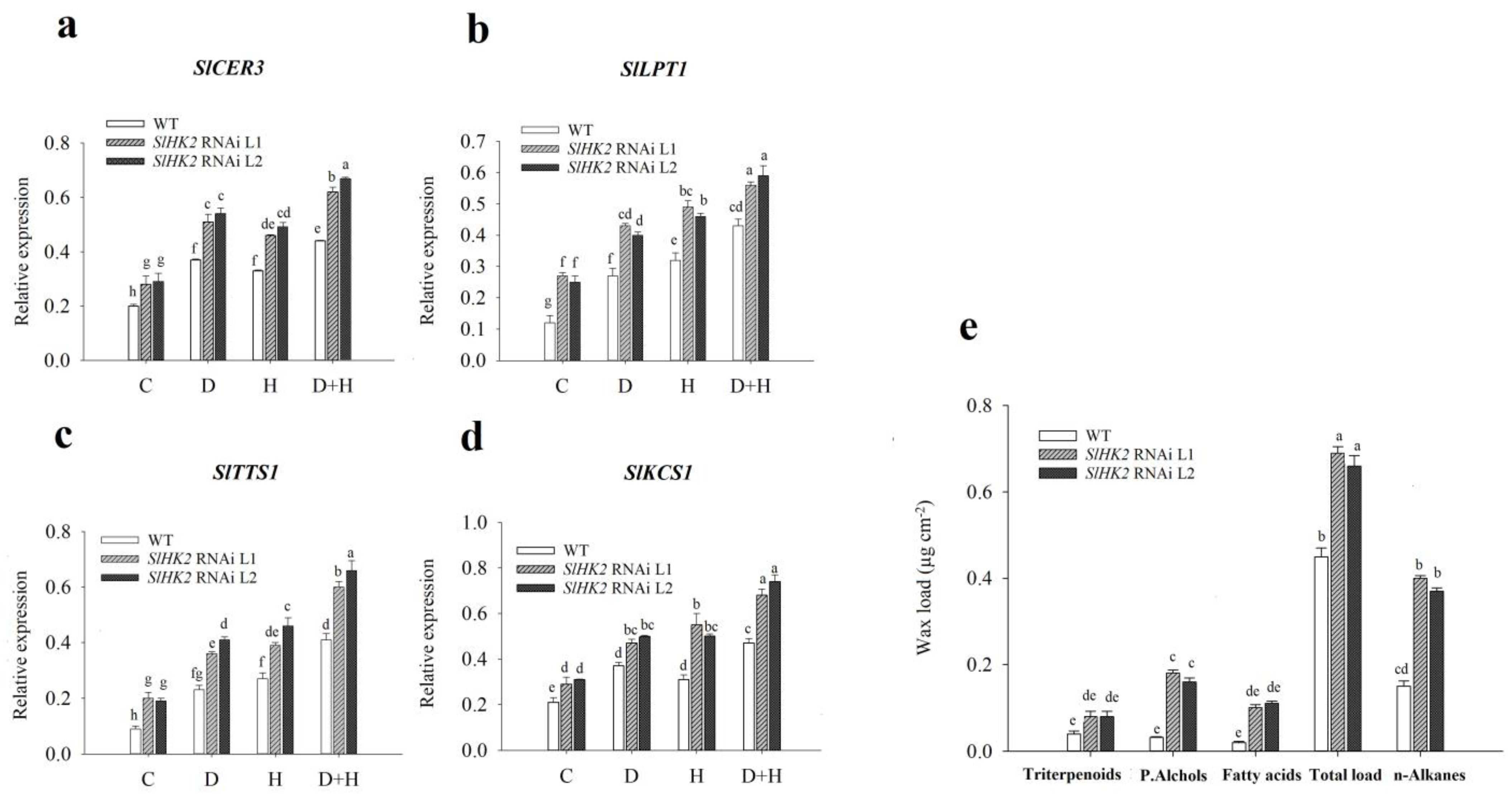
2.5. Transcript Quantification
2.6. Enzymatic Activities
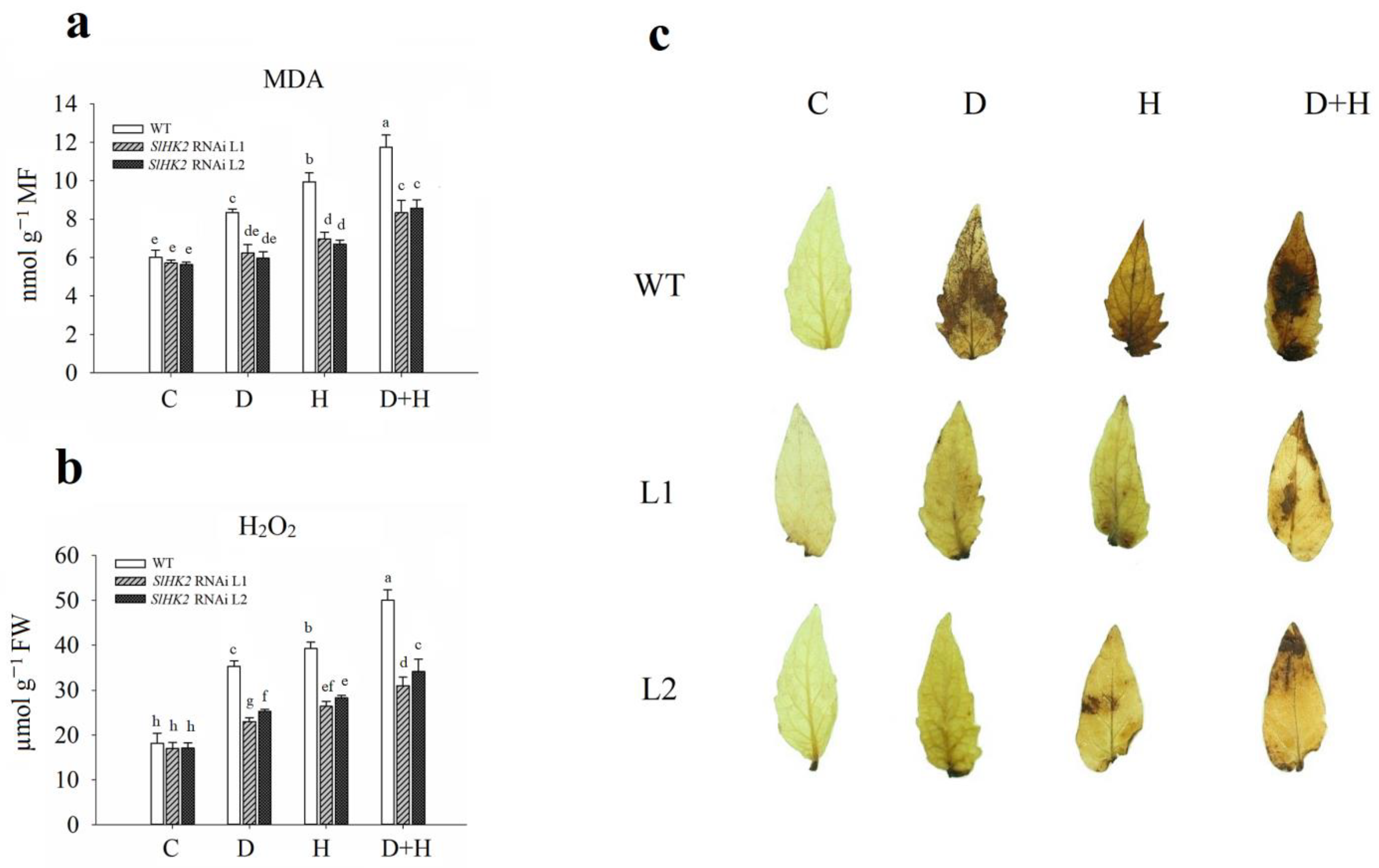
2.7. H2O2 Content and Lipid Peroxidation
2.8. DAB Staining for H2O2
2.9. Accumulation of Cuticular Wax
2.10. Relative Water Content
2.11. Electrolyte Leakage in Plants
2.12. Statistical Analysis
3. Results
3.1. SlHK2 RNAi Plants Are More Tolerant to Drought, Heat, and Combined Stresses than WT Plants
3.2. Photosynthesis and Gas Exchange of SlHK2 RNAi and WT Plants under Stress
3.3. Oxidative Stress Induced in SlHK2 RNAi and WT Plants under Drought, Heat, and Combined Stresses
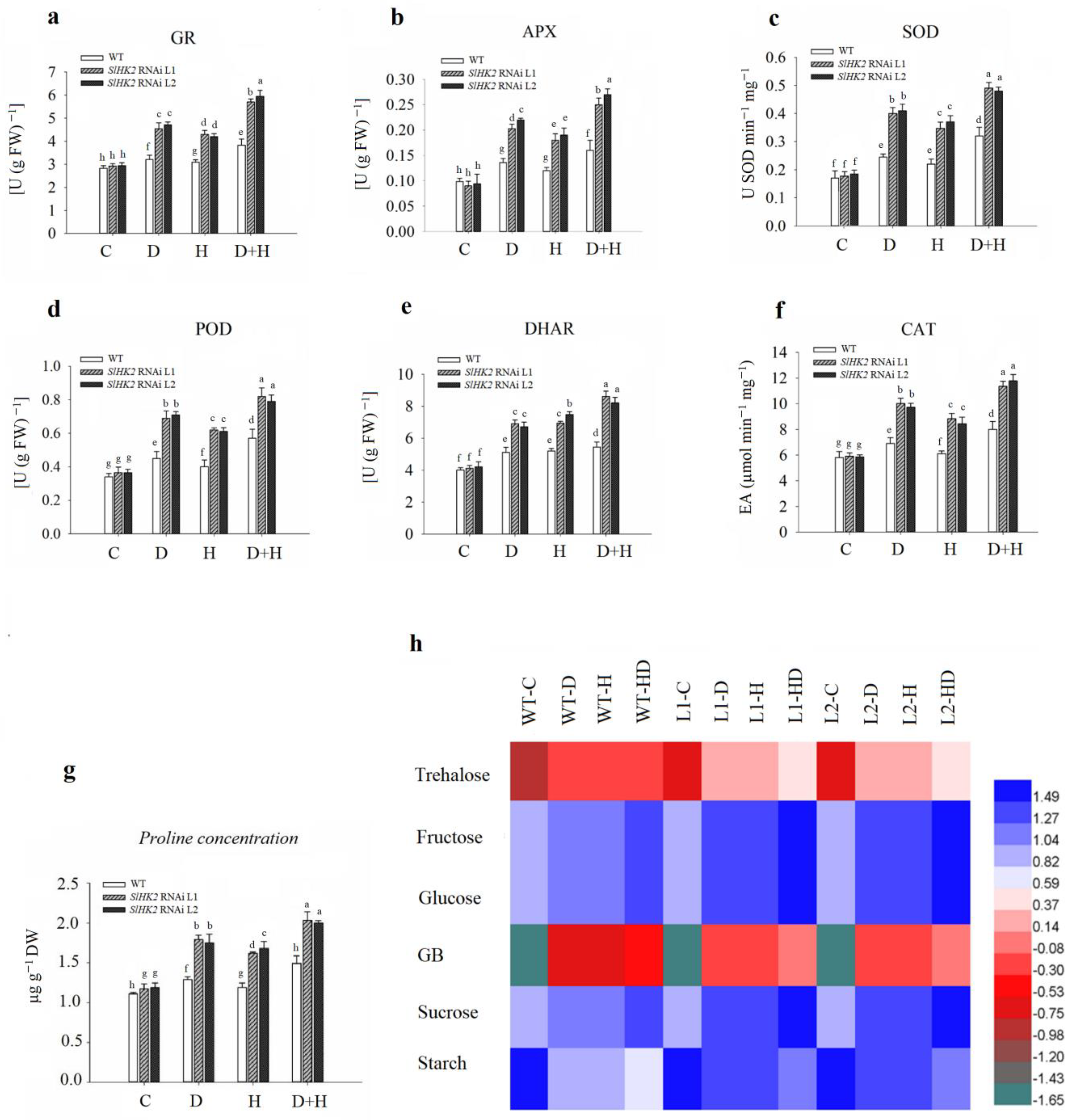
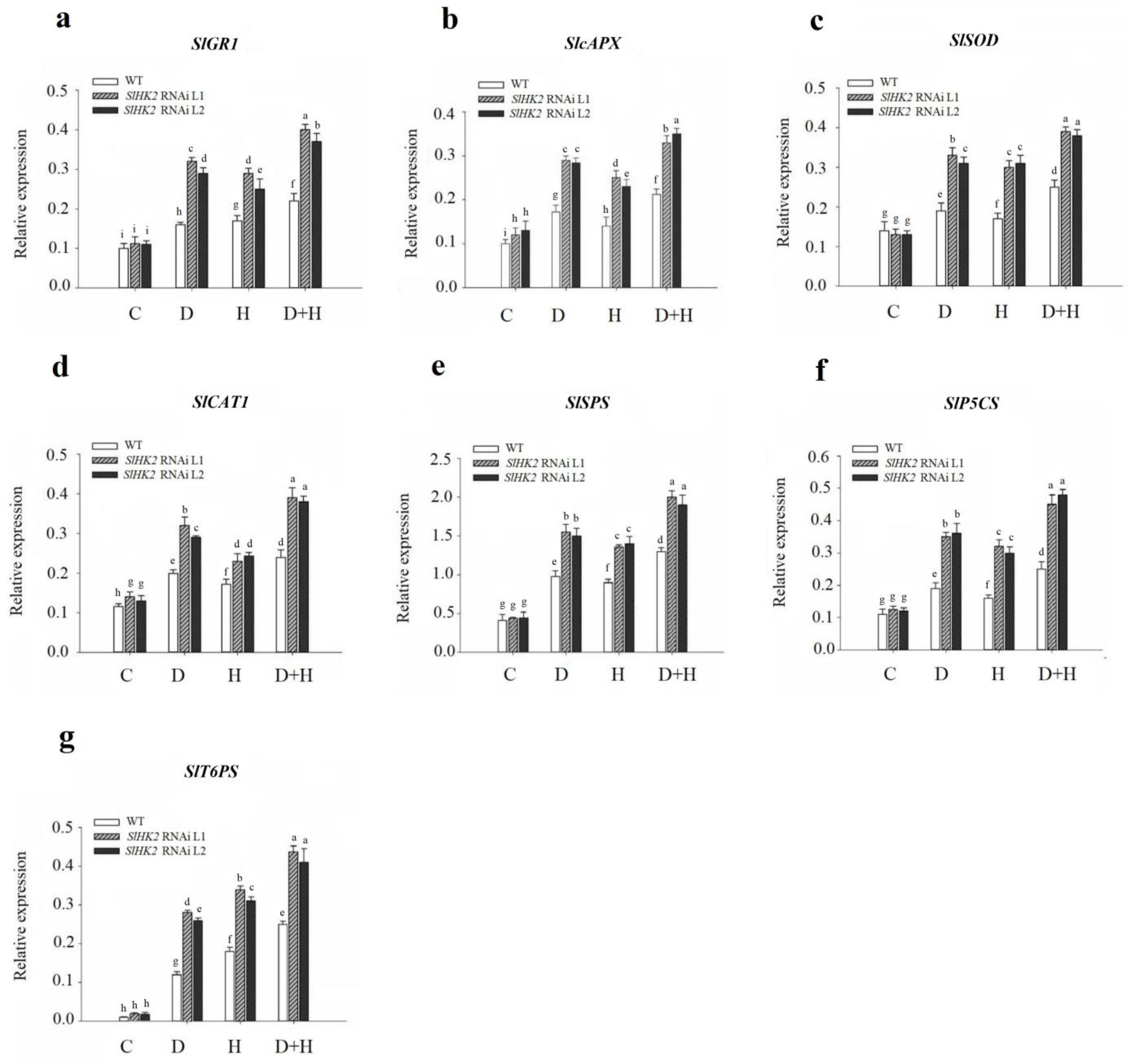
3.4. Antioxidant Defense System in SlHK2 RNAi and WT Plants under Stress
4. Discussion
Oxidative Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar]
- Elkelish, A.A.; Alhaithloul, H.A.S.; Qari, S.H.; Soliman, M.H.; Hasanuzzaman, M. Pretreatment with Trichoderma harzianum alleviates waterlogging-induced growth alterations in tomato seedlings by modulating physiological, biochemical, and molecular mechanisms. Environ. Exp. Bot. 2020, 171, 103946. [Google Scholar]
- Shaar-Moshe, L.; Blumwald, E.; Peleg, Z. Unique physiological and transcriptional shifts under combinations of salinity, drought, and heat. Plant Physiol. 2017, 174, 421. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Zhang, T.; Wu, Z.; Zhao, T. Unique miRNAs and their targets in tomato leaf responding to combined drought and heat stress. BMC Plant Biol. 2020, 20, 107. [Google Scholar] [CrossRef] [Green Version]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hu, T.; Fang, L.; Peng, X.; Liu, F. CO2 elevation modulates the response of leaf gas exchange to progressive soil drying in tomato plants. Agric. For. Meteorol. 2019, 268, 181–188. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Cvikrová, M.; Gemperlová, L.; Martincová, O.; Vanková, R. Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol. Biochem. 2013, 73, 7–15. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Cervilla, L.M.; Blasco, B.; Rios, J.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [Green Version]
- Stoop, J.M.H.; Williamson, J.D.; Mason Pharr, D. Mannitol metabolism in plants: A method for coping with stress. Trends Plant Sci. 1996, 1, 139–144. [Google Scholar] [CrossRef]
- Rai, G.K.; Rai, N.P.; Rathaur, S.; Kumar, S.; Singh, M. Expression of rd29A::AtDREB1A/CBF3 in tomato alleviates drought-induced oxidative stress by regulating key enzymatic and non-enzymatic antioxidants. Plant Physiol. Biochem. 2013, 69, 90–100. [Google Scholar] [CrossRef]
- Rai, A.C.; Singh, M.; Shah, K. Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants. Plant Physiol. Biochem. 2012, 61, 108–114. [Google Scholar] [CrossRef]
- Lu, H.; Xu, P.; Hu, K.; Xiao, Q.; Wen, J.; Yi, B.; Ma, C.; Tu, J.; Fu, T.; Shen, J. Transcriptome profiling reveals cytokinin promoted callus regeneration in Brassica juncea. Plant Cell Tissue Organ Cult. 2020, 141, 191–206. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book 2014, 12, e0168. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Herrera-Estrella, L.; Tran, L.S.P. The Yin–Yang of cytokinin homeostasis and drought acclimation/adaptation. Trends Plant Sci. 2016, 21, 548–550. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, N.Y.; Kang, N.Y.; Novák, O.; Ku, S.J.; Cho, C.; Lee, D.J.; Lee, E.J.; Strnad, M.; Kim, J. A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J. Biol. Chem. 2010, 285, 30. [Google Scholar] [CrossRef] [Green Version]
- Synkova, H.; Semoradova, S.; Schnablová, R.; Witters, E.; Husak, M.; Valcke, R. Cytokinin-induced activity of antioxidant enzymes in transgenic P ssu-ipt tobacco during plant ontogeny. Biol. Plant. 2006, 50, 31–41. [Google Scholar] [CrossRef]
- Yang, D.Q.; Luo, Y.L.; Dong, W.H.; Yin, Y.P.; Li, Y.; Wang, Z.L. Response of photosystem II performance and antioxidant enzyme activities in stay-green wheat to cytokinin. Photosynthetica 2018, 56, 567–577. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Ha, C.V.; Nishiyama, R.; Watanabe, Y.; Leyva-González, M.A.; Fujita, Y.; Tran, U.T.; Li, W.; Tanaka, M.; Seki, M.; et al. Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 3090–3095. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, C.; Ohashi, Y.; Sato, S.; Kato, T.; Tabata, S.; Ueguchi, C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004, 16, 1365. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.Y.; Cho, C.; Kim, N.Y.; Kim, J. Cytokinin receptor-dependent and receptor-independent pathways in the dehydration response of Arabidopsis thaliana. J. Plant Physiol. 2012, 169, 1382–1391. [Google Scholar] [CrossRef]
- Schmidt, R.; Willmitzer, L. High efficiency Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana leaf and cotyledon explants. Plant Cell Rep. 1988, 7, 583–586. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Blunden, C.A.; Wilson, M.F. A specific method for the determination of soluble sugars in plant extracts using enzymatic analysis and its application to the sugar content of developing pear fruit buds. Anal. Biochem. 1985, 151, 403–408. [Google Scholar] [CrossRef]
- Rasmussen, T.S.; Henry, R.J. Starch determination in horticultural plant material by an enzymic-colorimetric procedure. J. Sci. Food Agric. 1990, 52, 159–170. [Google Scholar] [CrossRef]
- Schulze, U.; Larsen, M.E.; Villadsen, J. Determination of intracellular trehalose and glycogen in Saccharomyces cerevisiae. Anal. Chem. 1995, 228, 143–149. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125. [Google Scholar] [CrossRef] [Green Version]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. Plant Physiol. 1977, 59, 315. [Google Scholar] [CrossRef] [Green Version]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B.N. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Dewdney, J.; Blee, K.A.; Stone, J.M.; Asai, T.; Plotnikov, J.; Denoux, C.; Hayes, T.; Gerrish, C.; Davies, D.R.; et al. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006, 47, 851–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Wang, M.; Wu, H.; Xu, J.; Li, C.; Wang, Y.; Wang, Z. Five fatty acyl-coenzyme a reductases are involved in the biosynthesis of primary alcohols in Aegilops tauschii leaves. Front Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Wang, G.; Wang, M.; Zhang, S. Exogenous melatonin improves tolerance to water deficit by promoting cuticle formation in tomato plants. Molecules 2018, 23, 1605. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Role of cytokinin responsive two-component system in ABA and osmotic stress signalings. Plant Signal. Behav. 2010, 5, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi Shinozaki, K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, R.; Watanabe, Y.; Leyva-Gonzalez, M.A.; Ha, C.V.; Fujita, Y.; Tanaka, M.; Seki, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Herrera-Estrella, L.; et al. Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. USA 2013, 110, 4840–4845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonhati, R.; Mello, S.C.; Momesso, P.; Pedroso, R.M. L-proline alleviates heat stress of tomato plants grown under protected environment. Sci. Hortic. 2020, 268, 109370. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically triggered heat and drought stress tolerance in rice by proline application. J. Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Kashiwagi, J.; Krishnamurthy, L.; Purushothaman, R.; Upadhyaya, H.D.; Gaur, P.M.; Gowda, C.L.L.; Ito, O.; Varshney, R.K. Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.). Field Crops Res. 2015, 170, 47–54. [Google Scholar] [CrossRef]
- Gagné-Bourque, F.; Bertrand, A.; Claessens, A.; Aliferis, K.A.; Jabaji, S. Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. Front Plant Sci. 2016, 7, 584. [Google Scholar] [CrossRef] [Green Version]
- Meister, R.; Rajani, M.S.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef]
- Macková, H.; Hronková, M.; Dobrá, J.; Turečková, V.; Novák, O.; Lubovská, Z.; Motyka, V.; Haisel, D.; Hájek, T.; Prášil, I.T. Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J. Exp. Bot. 2013, 64, 2805–2815. [Google Scholar] [CrossRef]
- Robbins, N.E.; Dinneny, J.R. Growth is required for perception of water availability to pattern root branches in plants. Proc. Natl. Acad. Sci. USA 2018, 115, E822–E831. [Google Scholar] [CrossRef] [Green Version]
- Schluepmann, H.; Pellny, T.; van Dijken, A.; Smeekens, S.; Paul, M. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6849–6854. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, H.; Takei, K.; Hirose, N. Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci. 2006, 11, 440–448. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Biswas, M.S.; Terada, R.; Mano, J. Inactivation of carbonyl-detoxifying enzymes by H2O2 Is a trigger to increase carbonyl load for initiating programmed cell death in plants. Antioxidants 2020, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sairam, R.K.; Srivastava, G.C.; Agarwal, S.; Meena, R.C. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant. 2005, 49, 85. [Google Scholar] [CrossRef]
- Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Oxidative stress in plants. Antioxidants 2020, 9, 481. [Google Scholar] [CrossRef]
- Pandey, A.K.; Gautam, A. Stress responsive gene regulation in relation to hydrogen sulfide in plants under abiotic stress. Physiol. Plant. 2020, 168, 511–525. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushtaq, N.; Wang, Y.; Fan, J.; Li, Y.; Ding, J. Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato. Plants 2022, 11, 154. https://doi.org/10.3390/plants11020154
Mushtaq N, Wang Y, Fan J, Li Y, Ding J. Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato. Plants. 2022; 11(2):154. https://doi.org/10.3390/plants11020154
Chicago/Turabian StyleMushtaq, Naveed, Yong Wang, Junmiao Fan, Yi Li, and Jing Ding. 2022. "Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato" Plants 11, no. 2: 154. https://doi.org/10.3390/plants11020154
APA StyleMushtaq, N., Wang, Y., Fan, J., Li, Y., & Ding, J. (2022). Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato. Plants, 11(2), 154. https://doi.org/10.3390/plants11020154





