New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects
Abstract
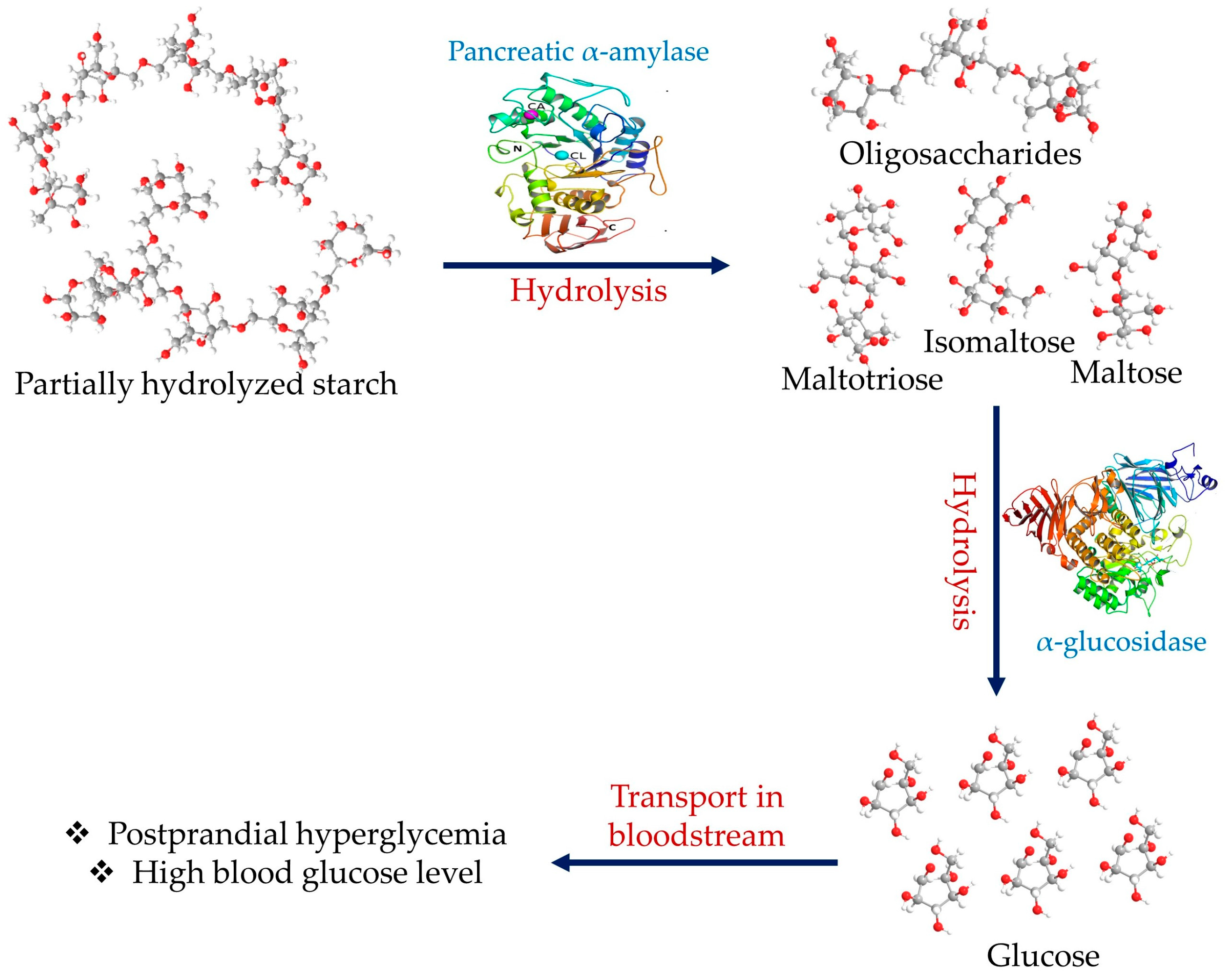
:1. Introduction
2. Alpha-Amylase Structure and Mechanism of Action
3. Plant Extracts as an α-Amylase Inhibitor Source
4. Secondary Metabolites Isolated from Various Plant Sources as Potential α-Amylase Inhibitors
4.1. Flavonoids
4.2. Terpenoids
4.3. Polysaccharides
4.4. Phenolic Acids
4.5. Tannins
4.6. Miscellaneous Secondary Metabolites as α-Amylase Inhibitors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and Treatment of Type 2 Diabetes: Perspectives on the Past, Present, and Future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharroubi, A.T. Diabetes Mellitus: The Epidemic of the Century. World J. Diabetes 2015, 6, 850. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Zeitler, P. Acute and Chronic Complications of Type 2 Diabetes Mellitus in Children and Adolescents. Lancet 2007, 369, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Association, A.D. Standards of Medical Care in Diabetes—2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.-H.; Stevens, G.A.; et al. National, Regional, and Global Trends in Fasting Plasma Glucose and Diabetes Prevalence since 1980: Systematic Analysis of Health Examination Surveys and Epidemiological Studies with 370 Country-Years and 2·7 Million Participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/idf_atlas_10th_edition_2021.pdf (accessed on 1 May 2023).
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Provisional Report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [Green Version]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, Vegetables, and Mushrooms for the Preparation of Extracts with α-Amylase and α-Glucosidase Inhibition Properties: A Review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.M.R.; Menichini, F. Natural Products as α-Amylase and & α-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Kawabata, J.; Kurihara, H.; Niki, R. Isolation and Identification of α -Glucosidase Inhibitors from Tochu-Cha (Eucommia Ulmoides). Biosci. Biotechnol. Biochem. 1997, 61, 177–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horii, S.; Fukase, H.; Matsuo, T.; Kameda, Y.; Asano, N.; Matsui, K. Synthesis and. Alpha.-D-Glucosidase Inhibitory Activity of N-Substituted Valiolamine Derivatives as Potential Oral Antidiabetic Agents. J. Med. Chem. 1986, 29, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Laar, F. Alpha-Glucosidase Inhibitors in the Early Treatment of Type 2 Diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Van de Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; Van de Lisdonk, E.H.; Rutten, G.E.; Van Weel, C. Alpha-Glucosidase Inhibitors for Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2005, 2009. [Google Scholar] [CrossRef] [Green Version]
- Ho, P.M.; Rumsfeld, J.S.; Masoudi, F.A.; McClure, D.L.; Plomondon, M.E.; Steiner, J.F.; Magid, D.J. Effect of Medication Nonadherence on Hospitalization and Mortality among Patients with Diabetes Mellitus. Arch. Intern. Med. 2006, 166, 1836. [Google Scholar] [CrossRef] [Green Version]
- Kashtoh, H.; Hussain, S.; Khan, A.; Saad, S.M.; Khan, J.A.J.; Khan, K.M.; Perveen, S.; Choudhary, M.I. Oxadiazoles and Thiadiazoles: Novel α-Glucosidase Inhibitors. Bioorganic Med. Chem. 2014, 22, 5454–5465. [Google Scholar] [CrossRef]
- Niaz, H.; Kashtoh, H.; Khan, J.A.J.; Khan, A.; Wahab, A.T.; Alam, M.T.; Khan, K.M.; Perveen, S.; Choudhary, M.I. Synthesis of Diethyl 4-Substituted-2,6-Dimethyl-1,4-Dihydropyridine-3,5-Dicarboxylates as a New Series of Inhibitors against Yeast α-Glucosidase. Eur. J. Med. Chem. 2015, 95, 199–209. [Google Scholar] [CrossRef]
- Kashtoh, H.; Muhammad, M.T.; Khan, J.J.A.; Rasheed, S.; Khan, A.; Perveen, S.; Javaid, K.; Atia-Tul-Wahab; Khan, K.M.; Choudhary, M.I. Dihydropyrano [2,3-c] Pyrazole: Novel in Vitro Inhibitors of Yeast α-Glucosidase. Bioorg. Chem. 2016, 65, 61–72. [Google Scholar] [CrossRef]
- Zhang, X.; World Health Organization. Traditional Medicine Strategy 2002–2005; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Al-Aboudi, A.; Afifi, F.U. Plants Used for the Treatment of Diabetes in Jordan: A Review of Scientific Evidence. Pharm. Biol. 2011, 49, 221–239. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Committee on Diabetes Mellitus [Meeting Held in Geneva from 25 September to 1 October 1979]: Second Report; World Health Organization: Geneva, Switzerland, 1980; ISBN 9241206462. [Google Scholar]
- Butterworth, P.J.; Warren, F.J.; Ellis, P.R. Human α-Amylase and Starch Digestion: An Interesting Marriage. Starch—Stärke 2011, 63, 395–405. [Google Scholar] [CrossRef]
- Patel, H.; Royall, P.G.; Gaisford, S.; Williams, G.R.; Edwards, C.H.; Warren, F.J.; Flanagan, B.M.; Ellis, P.R.; Butterworth, P.J. Structural and Enzyme Kinetic Studies of Retrograded Starch: Inhibition of α-Amylase and Consequences for Intestinal Digestion of Starch. Carbohydr. Polym. 2017, 164, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, B. Regional Distant Sequence Homology between Amylases, α-Glucosidases and Transglucanosylases. FEBS Lett. 1988, 230, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Henrissat, B. A Classification of Glycosyl Hydrolases Based on Amino Acid Sequence Similarities. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Plaunt, A.J.; de Lourdes Betancourt-Mendiola, M.; Smith, B.D. Biomolecule Recognition Using Transition Metal Ions and Hydrogen Bonding. In Comprehensive Supramolecular Chemistry II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 575–591. [Google Scholar] [CrossRef]
- Steer, M.L.; Levitzki, A. The Metal Specificity of Mammalian α-Amylase as Revealed by Enzyme Activity and Structural Probes. FEBS Lett. 1973, 31, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Vallee, B.L.; Stein, E.A.; Sumerwell, W.N.; Fischer, E.H. Metal Content of α-Amylases of Various Origins. J. Biol. Chem. 1959, 234, 2901–2905. [Google Scholar] [CrossRef]
- Levitzki, A.; Steer, M.L. The Allosteric Activation of Mammalian Alpha-Amylase by Chloride. Eur. J. Biochem. 1974, 41, 171–180. [Google Scholar] [CrossRef]
- Brayer, G.D.; Luo, Y.; Withers, S.G. The Structure of Human Pancreatic α -Amylase at 1.8 Å Resolution and Comparisons with Related Enzymes. Protein Sci. 1995, 4, 1730–1742. [Google Scholar] [CrossRef]
- Gumucio, D.L.; Wiebauer, K.; Caldwell, R.M.; Samuelson, L.C.; Meisler, M.H. Concerted Evolution of Human Amylase Genes. Mol. Cell. Biol. 1988, 8, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase Inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J. Pharm. Pharm. Sci. 2012, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Gao, H.; Tang, W.; Nie, S. Plant Non-Starch Polysaccharides That Inhibit Key Enzymes Linked to Type 2 Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2017, 1401, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Polyphenolic Compounds and Digestive Enzymes: In Vitro Non-Covalent Interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef] [Green Version]
- Brayer, G.D.; Sidhu, G.; Maurus, R.; Rydberg, E.H.; Braun, C.; Wang, Y.; Nguyen, N.T.; Overall, C.M.; Withers, S.G. Subsite Mapping of the Human Pancreatic α-Amylase Active Site through Structural, Kinetic, and Mutagenesis Techniques. Biochemistry 2000, 39, 4778–4791. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of Starch Digestion by α -Amylase—Structural Basis for Kinetic Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef]
- Truscheit, E.; Frommer, W.; Junge, B.; Müller, L.; Schmidt, D.D.; Wingender, W. Chemistry and Biochemistry of Microbialα-Glucosidase Inhibitors. Angew. Chemie Int. Ed. Engl. 1981, 20, 744–761. [Google Scholar] [CrossRef]
- Nichols, B.L.; Avery, S.; Sen, P.; Swallow, D.M.; Hahn, D.; Sterchi, E. The Maltase-Glucoamylase Gene: Common Ancestry to Sucrase-Isomaltase with Complementary Starch Digestion Activities. Proc. Natl. Acad. Sci. USA 2003, 100, 1432–1437. [Google Scholar] [CrossRef]
- Williamson, G. Possible Effects of Dietary Polyphenols on Sugar Absorption and Digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Ramasubbu, N.; Paloth, V.; Luo, Y.; Brayer, G.D.; Levine, M.J. Structure of Human Salivary α-Amylase at 1.6 Å Resolution: Implications for Its Role in the Oral Cavity. Acta Crystallogr. Sect. D Biol. Crystallogr. 1996, 52, 435–446. [Google Scholar] [CrossRef]
- Gilles, C.; Astier, J.; Marchis-Mouren, G.; Cambillau, C.; Payan, F. Crystal Structure of Pig Pancreatic Alpha-Amylase Isoenzyme II, in Complex with the Carbohydrate Inhibitor Acarbose. Eur. J. Biochem. 1996, 238, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, R.; Levitzki, A. Identity and Properties of the Chloride Effector Binding Site in Hog Pancreatic α-Amylase. Biochemistry 1976, 15, 1987–1993. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Janeček, Š.; Svensson, B. Relationship of Sequence and Structure to Specificity in the α-Amylase Family of Enzymes. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 2001, 1546, 1–20. [Google Scholar] [CrossRef]
- Schrödinger, L.L.C.; DeLano, W. Pymol. PyMOL Mol. Graph. Syst. Version 2020, 2. Available online: https://pymol.org/2/support.html? (accessed on 1 April 2022).
- Nahoum, V.; Roux, G.; Anton, V.; Rougé, P.; Puigserver, A.; Bischoff, H.; Henrissat, B.; Payan, F. Crystal Structures of Human Pancreatic α-Amylase in Complex with Carbohydrate and Proteinaceous Inhibitors. Biochem. J. 2000, 346, 201–208. [Google Scholar] [CrossRef]
- Aghajari, N.; Feller, G.; Gerday, C.; Haser, R. Structural Basis of α-Amylase Activation by Chloride. Protein Sci. 2002, 11, 1435–1441. [Google Scholar] [CrossRef]
- Qian, M.; Haser, R.; Buisson, G.; Duee, E.; Payan, F. The Active Center of a Mammalian .Alpha.-Amylase. Structure of the Complex of a Pancreatic. Alpha.-Amylase with a Carbohydrate Inhibitor Refined to 2.2-.ANG. Resolution. Biochemistry 1994, 33, 6284–6294. [Google Scholar] [CrossRef] [PubMed]
- Feller, G.; le Bussy, O.; Houssier, C.; Gerday, C. Structural and Functional Aspects of Chloride Binding to Alteromonas Haloplanctis α-Amylase. J. Biol. Chem. 1996, 271, 23836–23841. [Google Scholar] [CrossRef] [Green Version]
- Robyt, J.F. Starch: Structure, Properties, Chemistry, and Enzymology. In Glycoscience; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1437–1472. [Google Scholar] [CrossRef]
- Robyt, J.F.; French, D. Multiple Attack Hypothesis of α-Amylase Action: Action of Porcine Pancreatic, Human Salivary, and Aspergillus Oryzae α-Amylases. Arch. Biochem. Biophys. 1967, 122, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Seigner, C.; Prodanov, E.; Marchis-Mouren, G. The Determination of Subsite Binding Energies of Porcine Pancreatic α-Amylase by Comparing Hydrolytic Activity towards Substrates. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 1987, 913, 200–209. [Google Scholar] [CrossRef]
- Jarald, E.; Joshi, S.B.; Jain, D. Diabetes and Herbal Medicines. Pharmacol. Ther. 2008, 7, 97–106. [Google Scholar]
- Hamdan, I.; Afifi, F. Studies on the in Vitro and in Vivo Hypoglycemic Activities of Some Medicinal Plants Used in Treatment of Diabetes in Jordanian Traditional Medicine. J. Ethnopharmacol. 2004, 93, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Tag, H.; Kalita, P.; Dwivedi, P.; Das, A.K.; Namsa, N.D. Herbal Medicines Used in the Treatment of Diabetes Mellitus in Arunachal Himalaya, Northeast, India. J. Ethnopharmacol. 2012, 141, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Day, C. Traditional Plant Medicines as Treatments for Diabetes. Diabetes Care 1989, 12, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Baharvand-Ahmadi, B.; Bahmani, M.; Tajeddini, P.; Naghdi, N.; Rafieian-Kopaei, M. An Ethno-Medicinal Study of Medicinal Plants Used for the Treatment of Diabetes. J. Nephropathol. 2015, 5, 44–50. [Google Scholar] [CrossRef]
- Giovannini, P.; Howes, M.-J.R.; Edwards, S.E. Medicinal Plants Used in the Traditional Management of Diabetes and Its Sequelae in Central America: A Review. J. Ethnopharmacol. 2016, 184, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Moreira, D.D.L.; Teixeira, S.S.; Monteiro, M.H.D.; De-Oliveira, A.C.A.X.; Paumgartten, F.J.R. Traditional Use and Safety of Herbal Medicines. Rev. Bras. Farmacogn. 2014, 24, 248–257. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Daoudi, N.E.; Bouhrim, M.; Ouassou, H.; Legssyer, A.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M. Inhibitory Effect of Roasted/Unroasted Argania spinosa Seeds Oil on α- Glucosidase, α-Amylase and Intestinal Glucose Absorption Activities. S. Afr. J. Bot. 2020, 135, 413–420. [Google Scholar] [CrossRef]
- Quek, A.; Kassim, N.K.; Ismail, A.; Latif, M.A.M.; Shaari, K.; Tan, D.C.; Lim, P.C. Identification of Dipeptidyl Peptidase-4 and α-Amylase Inhibitors from Melicope glabra (Blume) TG Hartley (Rutaceae) Using Liquid Chromatography Tandem Mass Spectrometry, In Vitro and In Silico Methods. Molecules 2020, 26, 1. [Google Scholar] [CrossRef]
- Tekulu, G.H.; Araya, E.M.; Mengesha, H.G. In Vitro α-Amylase Inhibitory Effect of TLC Isolates of Aloe megalacantha Baker and Aloe monticola Reynolds. BMC Complement. Altern. Med. 2019, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, S.K.; Kumar, A.; Bhatt, R. Hedychium coronarium Rhizomes: Promising Antidiabetic and Natural Inhibitor of α-Amylase and α-Glucosidase. J. Diet. Suppl. 2020, 17, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Jaradat, N.; Elaraj, J.; Hamdan, A.; Lebdeh, S.A.; Halawa, T. Evaluation of the Hypoglycemic Effect of Seven Wild Folkloric Edible Plants from Palestine (Antidiabetic Effect of Seven Plants from Palestine). J. Complement. Integr. Med. 2020, 17, 1–10. [Google Scholar] [CrossRef]
- Sansenya, S.; Payaka, A.; Wannasut, W.; Hua, Y.; Chumanee, S. Biological Activity of Rice Extract and the Inhibition Potential of Rice Extract, Rice Volatile Compounds and Their Combination against α-Glucosidase, α-Amylase and Tyrosinase. Int. J. Food Sci. Technol. 2021, 56, 1865–1876. [Google Scholar] [CrossRef]
- Xu, S.; Qin, L.; Mazhar, M.; Zhu, Y. Functional Components Profile and Glycemic Index of Kidney Beans. Front. Nutr. 2022, 9, 1044427. [Google Scholar] [CrossRef] [PubMed]
- Gök, H.N.; Deliorman Orhan, D.; Gürbüz, İ.; Aslan, M. Activity-Guided Isolation of α-Amylase, α-Glucosidase, and Pancreatic Lipase Inhibitory Compounds from Rhus coriaria L. J. Food Sci. 2020, 85, 3220–3228. [Google Scholar] [CrossRef] [PubMed]
- Quek, A.; Kassim, N.K.; Lim, P.C.; Tan, D.C.; Mohammad Latif, M.A.; Ismail, A.; Shaari, K.; Awang, K. α-Amylase and Dipeptidyl Peptidase-4 (DPP-4) Inhibitory Effects of Melicope latifolia Bark Extracts and Identification of Bioactive Constituents Using in Vitro and in Silico Approaches. Pharm. Biol. 2021, 59, 964–973. [Google Scholar] [CrossRef]
- Sani, D.H.; Munna, A.N.; Alam, M.J.; Salim, M.; Alam, M.J. Evaluation of α-Amylase Inhibition and Cytotoxic Activities of the Arachis hypogaea and Cinnamomum tamala. Curr. Nutr. Food Sci. 2020, 17, 328–336. [Google Scholar] [CrossRef]
- Romero Rocamora, C.; Ramasamy, K.; Meng Lim, S.; Majeed, A.B.A.; Agatonovic-Kustrin, S. HPTLC Based Approach for Bioassay-Guided Evaluation of Antidiabetic and Neuroprotective Effects of Eight Essential Oils of the Lamiaceae Family Plants. J. Pharm. Biomed. Anal. 2020, 178, 112909. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Q.; Wu, Y.; Ouyang, J. Inhibitory Effects of Acorn (Quercus variabilis Blume) Kernel-Derived Polyphenols on the Activities of α-Amylase, α-Glucosidase, and Dipeptidyl Peptidase IV. Food Biosci. 2021, 43, 101224. [Google Scholar] [CrossRef]
- Sen, A.; Kurkcuoglu, M.; Yildirim, A.; Senkardes, I.; Bitis, L.; Baser, K.H.C. Chemical Composition, Antiradical, and Enzyme Inhibitory Potential of Essential Oil Obtained from Aerial Part of Centaurea pterocaula Trautv. J. Essent. Oil Res. 2021, 33, 44–52. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Albu, C.; Savin, S.; Radu, G.L. In Vitro Evaluation of Antidiabetic and Anti-Inflammatory Activities of Polyphenolic-Rich Extracts from Anchusa officinalis and Melilotus officinalis. ACS Omega 2020, 5, 13014–13022. [Google Scholar] [CrossRef] [PubMed]
- Hoang Anh, L.; Xuan, T.D.; Dieu Thuy, N.T.; Van Quan, N.; Trang, L.T. Antioxidant and α-Amylase Inhibitory Activities and Phytocompounds of Clausena indica Fruits. Medicines 2020, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Govindasamy, J.; Nyola, N.K. In-Vitro and in-Vivo Anti-Hyperglycemic Potential of Prosopis cineraria Pods Extract and Fractions. J. Biol. Act. Prod. Nat. 2019, 9, 135–140. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Raja, K.; Vasanth, K.; Khusro, A.; Sadhasivam, S.; Sahibzada, M.U.K.; Gawwad, M.R.A.; Al Farraj, D.A.; Elshikh, M.S. Phytochemical Screening and in Vitro Antibacterial, Antioxidant, Anti-Inflammatory, Anti-Diabetic, and Wound Healing Attributes of Senna auriculata (L.) Roxb. Leaves. Arab. J. Chem. 2021, 14, 103345. [Google Scholar] [CrossRef]
- Al-Ahmed, A.; Khalil, H.E. Antidiabetic Activity of Terfeziaclaveryi; An in Vitro and in Vivo Study. Biomed. Pharmacol. J. 2019, 12, 603–608. [Google Scholar] [CrossRef]
- Prakash, V. Determination of α-Amylase Inhibitory Potential of Leaf Extracts of Rhododendron arboreum Sm. and Rhododendron campanulatum D. Don. J. Drug Deliv. Ther. 2022, 12, 20–22. [Google Scholar] [CrossRef]
- Mazumder, K.; Sumi, T.S.; Golder, M.; Biswas, B.; Maknoon; Kerr, P.G. Antidiabetic Profiling, Cytotoxicity and Acute Toxicity Evaluation of Aerial Parts of Phragmites karka (Retz.). J. Ethnopharmacol. 2021, 270, 113781. [Google Scholar] [CrossRef]
- da Silva, D.H.A.; Barbosa, H.d.M.; Beltrão, R.L.d.A.; Silva, C.d.F.O.; Moura, C.A.; Castro, R.N.; Almeida, J.R.G.d.S.; Gomes, D.A.; Lira, E.C. Hexane Fraction from Brazilian Morus nigra Leaves Improved Oral Carbohydrate Tolerance and Inhibits α-Amylase and α-Glucosidase Activities in Diabetic Mice. Nat. Prod. Res. 2021, 35, 4785–4788. [Google Scholar] [CrossRef]
- Ktari, N.; Ben Slama-Ben Salem, R.; Bkhairia, I.; Ben Slima, S.; Nasri, R.; Ben Salah, R.; Nasri, M. Functional Properties and Biological Activities of Peptides from Zebra Blenny Protein Hydrolysates Fractionated Using Ultrafiltration. Food Biosci. 2020, 34, 100539. [Google Scholar] [CrossRef]
- Renganathan, S.; Manokaran, S.; Vasanthakumar, P.; Singaravelu, U.; Kim, P.S.; Kutzner, A.; Heese, K. Phytochemical Profiling in Conjunction with in Vitro and in Silico Studies to Identify Human α-Amylase Inhibitors in Leucaena leucocephala (Lam.) de Wit for the Treatment of Diabetes Mellitus. ACS Omega 2021, 6, 19045–19057. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.P.; Pradhan, S.P.; Adhikari, K.; Nepal, S. Bergenia pacumbis from Nepal, an Astonishing Enzymes Inhibitor. BMC Complement. Med. Ther. 2020, 20, 198. [Google Scholar] [CrossRef]
- Jaradat, N.; Abualhasan, M.N.; Qadi, M.; Issa, L.; Mousa, A.; Allan, F.; Hindi, M.; Alhrezat, Z. Antiamylase, Antilipase, Antimicrobial, and Cytotoxic Activity of Nonea obtusifolia (Willd.) Dc. From Palestine. Biomed Res. Int. 2020, 2020, 8821319. [Google Scholar] [CrossRef] [PubMed]
- Jan, B.; Zahiruddin, S.; Basist, P.; Irfan, M.; Abass, S.; Ahmad, S. Metabolomic Profiling and Identification of Antioxidant and Antidiabetic Compounds from Leaves of Different Varieties of Morus alba Linn Grown in Kashmir. ACS Omega 2022, 7, 24317–24328. [Google Scholar] [CrossRef]
- Omar, R.M.; Badria, F.A.; Galala, A.A. An Emerging Flavone Glycoside from Phyllanthus emblica L.: As Promiscuous Enzyme Inhibitor and Potential Therapeutic in Chronic Diseases. S. Afr. J. Bot. 2023, 153, 290–296. [Google Scholar] [CrossRef]
- Timalsina, D.; Bhusal, D.; Devkota, H.P.; Pokhrel, K.P.; Sharma, K.R. α -Amylase Inhibitory Activity of Catunaregam spinosa (Thunb.) Tirveng.: In Vitro and in Silico Studies. Biomed Res. Int. 2021, 2021, 4133876. [Google Scholar] [CrossRef]
- Choudhary, N.; Prabhakar, P.K.; Khatik, G.L.; Chamakuri, S.R.; Tewari, D.; Suttee, A. Evaluation of Acute Toxicity, in-Vitro, in-Vivo Antidiabetic Potential of the Flavonoid Fraction of the Plant Chenopodium album L. Pharmacogn. J. 2021, 13, 765–779. [Google Scholar] [CrossRef]
- Saraswathi, K.; Bharkavi, R.; Khusro, A.; Sivaraj, C.; Arumugam, P.; Alghamdi, S.; Dablool, A.S.; Almehmadi, M.; Bannunah, A.M.; Umar Khayam Sahibzada, M. Assessment on in Vitro Medicinal Properties and Chemical Composition Analysis of Solanum virginianum Dried Fruits. Arab. J. Chem. 2021, 14, 103442. [Google Scholar] [CrossRef]
- Bello, M.; Jiddah-kazeem, B.; Fatoki, T.H.; Ibukun, E.O.; Akinmoladun, A.C. Antioxidant Property of Eucalyptus globulus Labill. Extracts and Inhibitory Activities on Carbohydrate Metabolizing Enzymes Related to Type-2 Diabetes. Biocatal. Agric. Biotechnol. 2021, 36, 102111. [Google Scholar] [CrossRef]
- Mikailu, S.; Okorafor, M.C.; Abo, K.A. Alpha-Amylase Inhibition and Membrane Stabilizing Effect of the Stem Bark of Maesobotrya dusenii Hutchinson. Int. J. Pharm. Sci. Res. 2019, 10, 5154–5159. [Google Scholar] [CrossRef]
- Hassan, A.; Khan Mohmand, N.Z.; Ullah, H.; Hussain, A. Antioxidant, Antidiabetic, and Antihypertension Inhibitory Potentials of Phenolic Rich Medicinal Plants. J. Chem. 2022, 2022, 9046780. [Google Scholar] [CrossRef]
- Remok, F.; Saidi, S.; Gourich, A.A.; Zibouh, K.; Maouloua, M.; El Makhoukhi, F.; El Menyiy, N.; Touijer, H.; Bouhrim, M.; Sahpaz, S.; et al. Phenolic Content, Antioxidant, Antibacterial, Antihyperglycemic, and α-Amylase Inhibitory Activities of Aqueous Extract of Salvia lavandulifolia Vahl. Pharmaceuticals 2023, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Karray, A.; Alonazi, M.; Jallouli, R.; Alanazi, H.; Ben Bacha, A. A Proteinaceous Alpha-Amylase Inhibitor from Moringa oleifera Leaf Extract: Purification, Characterization, and Insecticide Effects against C. Maculates Insect Larvae. Molecules 2022, 27, 4222. [Google Scholar] [CrossRef] [PubMed]
- Ukwuani-Kwaja, A.; Sani, I.; Sylvester, M.N.N.; Kabir, A.O.; Abolaji, O.K.; Ukwuani-Kwaja, A.; Sani, I.; Sylvester, M.N.N. In-Vitro Antidiabetic Effect of Ziziphus mucronata Leave Extracts. J. Drug Deliv. Ther. 2021, 11, 9–13. [Google Scholar] [CrossRef]
- Olaokun, O.O.; Manonga, S.A.; Zubair, M.S.; Maulana, S.; Mkolo, N.M. Molecular Docking and Molecular Dynamics Studies of Antidiabetic Phenolic Compound Isolated from Leaf Extract of Englerophytum magalismontanum (Sond.) T.D.Penn. Molecules 2022, 27, 3175. [Google Scholar] [CrossRef] [PubMed]
- Yashoda, K.; Deegendra, K.; Bimala, S. Antioxidant, Ptp 1B Inhibition and A-Amylase Inhibition Property and Gc-Ms Analysis of Methanolic Leaves Extract of Achyranthes aspera and Catharanthus roseus of Nepal. Int. J. Pharm. Pharm. Sci. 2021, 13, 49–55. [Google Scholar] [CrossRef]
- Irfan Dar, M.; Qureshi, M.I.; Zahiruddin, S.; Abass, S.; Jan, B.; Sultan, A.; Ahmad, S. In Silico Analysis of PTP1B Inhibitors and TLC-MS Bioautography-Based Identification of Free Radical Scavenging and α-Amylase Inhibitory Compounds from Heartwood Extract of Pterocarpus marsupium. ACS Omega 2022, 7, 46156–46173. [Google Scholar] [CrossRef]
- Li, F.; Luo, T.; Hou, J.; Fei, T.; Zhang, J.; Wang, L. Natural α-Glucosidase and α-Amylase Inhibitors from Raspberry (Rubus corchorifolius L.) Leaf-Tea: Screening, Identification and Molecular Docking Analysis. Lwt 2023, 181, 114763. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P.; Huang, Z.; Zhao, Z. Phenolics from Sterculia nobilis Smith Pericarp By-Products Delay Carbohydrate Digestion by Uncompetitively Inhibiting α-Glucosidase and α-Amylase. LWT 2023, 173, 114339. [Google Scholar] [CrossRef]
- Sakhr, K.; El Khatib, S. Physiochemical Properties and Medicinal, Nutritional and Industrial Applications of Lebanese Sumac (Syrian Sumac—Rhus coriaria): A Review. Heliyon 2020, 6, e03207. [Google Scholar] [CrossRef] [Green Version]
- Pleşca-Manea, L.; Pârvu, A.E.; Pârvu, M.; Taămaş, M.; Buia, R.; Puia, M. Effects of Melilotus officinalis on Acute Inflammation. Phyther. Res. 2002, 16, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Burlando, B.; Verotta, L.; Cornara, L.; Bottini-Massa, E. Herbal Principles in Cosmetics: Properties and Mechanisms of Action; CRC Press: Boca Raton, FL, USA, 2010; ISBN 1439812144. [Google Scholar]
- Bakhshayeshi, S.; Madani, S.; Hemmatabadi, M.; Heshmat, R.; Larijani, B. Effects of Semelil (ANGIPARSTM) on Diabetic Peripheral Neuropathy: A Randomized, Double-Blind Placebo-Controlled Clinical Trial. Daru 2011, 19, 65–70. [Google Scholar]
- Nithya, M.; Ragavendran, C.; Natarajan, D. Antibacterial and Free Radical Scavenging Activity of a Medicinal Plant Solanum Xanthocarpum. Int. J. Food Prop. 2018, 21, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Tekuri, S.K.; Pasupuleti, S.K.; Konidala, K.K.; Amuru, S.R.; Bassaiahgari, P.; Pabbaraju, N. Phytochemical and Pharmacological Activities of Solanum Surattense Burm. f.–A Review. J. Appl. Pharm. Sci. 2019, 9, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.P.A.; Lauretti, L.B.C.; Alvarenga, V.O.; Paulino, B.N.; Angolini, C.F.F.; Neri-Numa, I.A.; Orlando, E.A.; Pallone, J.A.L.; Sant’Ana, A.S.; Pastore, G.M. Evaluation of Fruta-Do-Lobo (Solanum Lycocarpum St. Hill) Starch on the Growth of Probiotic Strains. Food Res. Int. 2020, 133, 109187. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.A.; Fernando Figueiredo Angolini, C.; de Souza-Sporkens, J.C.; da Silva, T.A.; Coutinho Franco de Oliveira, H.; Pastore, G.M. Brazilian Sunberry (Solanum Oocarpum Sendtn): Alkaloid Composition and Improvement of Mitochondrial Functionality and Insulin Secretion of INS-1E Cells. Food Res. Int. 2021, 148, 110589. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-QTOF-MS/MS Characterization, Antioxidant Activities and Inhibitory Ability of Digestive Enzymes with Molecular Docking Analysis of Various Parts of Raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Sun, P.; Wang, R.; Zhao, X. Gastroprotective Effects of Methanolic Extract of S Terculia Nobilis Smith Seeds in Reserpine-Induced Gastric Ulcer in Mice. J. Food Biochem. 2015, 39, 230–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, R.; Mo, Y.; Li, Q.; Lin, W.; Yuan, G. Colletotrichum Siamense: A Novel Leaf Pathogen of Sterculia nobilis Smith Detected in China. For. Pathol. 2020, 50, e12575. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Li, Y.; Lin, S.-J.; Li, H.-B. Green Extraction of Natural Antioxidants from the Sterculia nobilis Fruit Waste and Analysis of Phenolic Profile. Molecules 2018, 23, 1059. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Garg, V.; Paul, A. Antihyperglycemic, Antihyperlipidemic and Antioxidative Potential of Prosopis cineraria Bark. Indian J. Clin. Biochem. 2010, 25, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Kant, S. Pharmacological Evaluation of Antidiabetic and Antihyperlipidemic Activity of Chenopodium album Root Extract in Male Wistar Albino Rat Models. Int. J. Green Pharm. 2018, 12, 115–122. [Google Scholar] [CrossRef]
- Odhav, B.; Kandasamy, T.; Khumalo, N.; Baijnath, H. Screening of African Traditional Vegetables for Their Alpha-Amylase Inhibitory Effect. J. Med. Plants Res 2010, 4, 1502–1507. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez, C.; Moñino, M.I.; Lax, V.; Quílez, M.; Sotomayor, J.A. Chemical Characterization of Salvia lavandulifolia Subsp. Vellerea in South-Eastern Spain. Acta Hortic. 2009, 317–324. [Google Scholar] [CrossRef]
- Zrira, S.; Menut, C.; Bessiere, J.M.; Elamrani, A.; Benjilali, B. A Study of the Essential Oil of Salvia lavandulifolia Vahl from Morocco. J. Essent. Oil Bear. Plants 2004, 7, 232–238. [Google Scholar] [CrossRef]
- Zarzuelo, A.; Risco, S.; Gámez, M.J.; Jimenez, J.; Cámara, M.; Martinez, M.A. Hypoglycemic Action of Vahl. ssp.: A Contribution to Studies of the Mechanism of Action. Life Sci. 1990, 47, 909–915. [Google Scholar] [CrossRef]
- Kabach, I.; Bouchmaa, N.; Zouaoui, Z.; Ennoury, A.; El Asri, S.; Laabar, A.; Oumeslakht, L.; Cacciola, F.; El Majdoub, Y.O.; Mondello, L.; et al. Phytochemical Profile and Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Activities of Oxalis pes-caprae Extracts in Alloxan-Induced Diabetic Mice. Biomed. Pharmacother. 2023, 160, 114393. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Sharif, A.; Zubair, H.M.; Akhtar, B.; Mobashar, A. In Vitro, In Silico, and In Vivo Studies of Cardamine hirsuta Linn as a Potential Antidiabetic Agent in a Rat Model. ACS Omega 2023, 8, 22623–22636. [Google Scholar] [CrossRef]
- Wan, P.; Cai, B.; Chen, H.; Chen, D.; Zhao, X.; Yuan, H.; Huang, J.; Chen, X.; Luo, L.; Pan, J. Antidiabetic Effects of Protein Hydrolysates from Trachinotus ovatus and Identification and Screening of Peptides with α-Amylase and DPP-IV Inhibitory Activities. Curr. Res. Food Sci. 2023, 6, 100446. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Zahoor, M.; Ayaz, M.; Ashraf, M.; Nawaz, A.; Alotaibi, A. Anti-Diabetic Potentials of Sorbaria tomentosa Lindl. Rehder: Phytochemistry (GC-MS Analysis), α-Amylase, α-Glucosidase Inhibitory, in Vivo Hypoglycemic, and Biochemical Analysis. Open Chem. 2023, 21, 20220339. [Google Scholar] [CrossRef]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-Salahi, R.; Nasr, F.A.; Bnouham, M.; et al. Artemisia absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef]
- Bouknana, S.; Daoudi, N.E.; Bouhrim, M.; Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Bnouham, M. Ammodaucus leucotrichus Coss. & Durieu: Antihyperglycemic Activity via the Inhibition of α-Amylase, α-Glucosidase, and Intestinal Glucose Absorption Activities and Its Chemical Composition. J. Pharm. Pharmacogn. Res. 2022, 10, 94–103. [Google Scholar] [CrossRef]
- Zeng, A.; Yang, R.; Yu, S.; Zhao, W. A Novel Hypoglycemic Agent: Polysaccharides from Laver (Porphyra spp.). Food Funct. 2020, 11, 9048–9056. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Gao, W.; Li, X.; Luo, S.; Wu, D.; Chye, F.Y. Garlic (Allium sativum L.) Polysaccharide Ameliorates Type 2 Diabetes Mellitus (T2DM) via the Regulation of Hepatic Glycogen Metabolism. NFS J. 2023, 31, 19–27. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Tran, N.N.; Pham, L.D.; Lai, N.V.; Ngoc, B. Flavonoids as Dual- Target Inhibitors against α—Glucosidase and α -Amylase: A Systematic Review of in Vitro Studies. Biol. Med. Chem. 2023, 1–41. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic Effects of Natural Plant Extracts via Inhibition of Carbohydrate Hydrolysis Enzymes with Emphasis on Pancreatic Alpha Amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-Amylase and α-Glucosidase: Potential Linkage for Whole Cereal Foods on Prevention of Hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Sivalingam, S.; Kandhasamy, S.; Chandrasekaran, S.; Vijayan, K.; Jacob, J.P.; Perumal, A.; Vijayakumar, S. A Flavone Derivative from Andrographis Echioides Leaf Extract Positively Alters the Molecular Targets of Insulin Signaling Pathway. S. Afr. J. Bot. 2022, 146, 760–770. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Zeng, W.; Tian, J.; Zhao, X.; Han, J.; Huang, D.; Gu, D. A Strategy Based on Liquid-Liquid-Refining Extraction and High-Speed Counter-Current Chromatography for the Bioassay-Guided Separation of Active Compound from Taraxacum Mongolicum. J. Chromatogr. A 2020, 1614, 460727. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, J.; Yuan, E.; Chen, J.; Zhang, Q.; Wang, Z.; Yin, Z. Extraction, Identification, and Starch-Digestion Inhibition of Phenolics from Euryale Ferox Seed Coat. J. Sci. Food Agric. 2023, 103, 3437–3446. [Google Scholar] [CrossRef]
- Sahnoun, M.; Bejar, S.; Daoud, L.; Ayadi, L.; Brini, F.; Saibi, W. Effect of Agave americana L. on the Human, and Aspergillus Oryzae S2 α-Amylase Inhibitions. Nat. Prod. Res. 2019, 33, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. A New Flavone Glucoside Together with Known Ellagitannins and Flavones with Anti-Diabetic and Anti-Obesity Activities from the Flowers of Pomegranate (Punica granatum). Nat. Prod. Res. 2019, 33, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Omar, A.M.; El-Araby, M.E.; Mass, S.; Ibrahim, S.R.M. Assessments of Alpha-Amylase Inhibitory Potential of Tagetes Flavonoids through In Vitro, Molecular Docking, and Molecular Dynamics Simulation Studies. Int. J. Mol. Sci. 2023, 24, 10195. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Koubaa, I.; Dhouib, I.; Khemakhem, B.; Marchand, P.; Allouche, N. New Specific A—Glucosidase Inhibitor Flavonoid from Thymelaea Tartonraira Leaves: Structure Elucidation, Biological and Molecular Docking Studies. Chem. Biodivers. 2023, 20, e202200944. [Google Scholar] [CrossRef]
- Luyen, N.T.; Binh, P.T.; Tham, P.T.; Hung, T.M.; Dang, N.H.; Dat, N.T.; Thao, N.P. Wedtrilosides A and B, Two New Diterpenoid Glycosides from the Leaves of Wedelia trilobata (L.) Hitchc. with α-Amylase and α-Glucosidase Inhibitory Activities. Bioorg. Chem. 2019, 85, 319–324. [Google Scholar] [CrossRef]
- Etsassala, N.G.E.R.; Badmus, J.A.; Marnewick, J.L.; Iwuoha, E.I.; Nchu, F.; Hussein, A.A. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities, Molecular Docking, and Antioxidant Capacities of Salvia Aurita Constituents. Antioxidants 2020, 9, 1149. [Google Scholar] [CrossRef]
- Mohammed, A.; Victoria Awolola, G.; Ibrahim, M.A.; Anthony Koorbanally, N.; Islam, M.S. Oleanolic Acid as a Potential Antidiabetic Component of Xylopia Aethiopica (Dunal) A. Rich. (Annonaceae) Fruit: Bioassay Guided Isolation and Molecular Docking Studies. Nat. Prod. Res. 2021, 35, 788–791. [Google Scholar] [CrossRef]
- Verma, A.; Pathak, P.; Rimac, H.; Khalilullah, H.; Kumar, V.; Grishina, M.; Potemkin, V.; Ahmed, B. A Triterpene Glochidon from Phyllanthus Debilis: Isolation, Computational Studies, and Antidiabetic Activity Evaluation. Biocatal. Agric. Biotechnol. 2021, 36, 102138. [Google Scholar] [CrossRef]
- Monzón Daza, G.; Meneses Macías, C.; Forero, A.M.; Rodríguez, J.; Aragón, M.; Jiménez, C.; Ramos, F.A.; Castellanos, L. Identification of α-Amylase and α-Glucosidase Inhibitors and Ligularoside A, a New Triterpenoid Saponin from Passiflora Ligularis Juss (Sweet granadilla) Leaves, by a Nuclear Magnetic Resonance-Based Metabolomic Study. J. Agric. Food Chem. 2021, 69, 2919–2931. [Google Scholar] [CrossRef]
- Uddin, M.J.; Russo, D.; Haque, M.A.; Çiçek, S.S.; Sönnichsen, F.D.; Milella, L.; Zidorn, C. Bioactive Abietane-Type Diterpenoid Glycosides from Leaves of Clerodendrum infortunatum (Lamiaceae). Molecules 2021, 26, 4121. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, W.; Zhang, Y.; Shi, L.; Xu, Z.; Xia, W.; Zhang, W. Structure Characteristics, Hypoglycemic and Immunomodulatory Activities of Pectic Polysaccharides from Rosa Setate x Rosa Rugosa Waste. Carbohydr. Polym. 2021, 253, 117190. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Guo, Y.; Chen, X.; Cui, D.; Wang, M.; Yang, W.; Chen, F. Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia Grosvenorii. Molecules 2022, 27, 4192. [Google Scholar] [CrossRef]
- Quan, N.; Wang, Y.D.; Li, G.R.; Liu, Z.Q.; Feng, J.; Qiao, C.L.; Zhang, H.F. Ultrasound–Microwave Combined Extraction of Novel Polysaccharide Fractions from Lycium Barbarum Leaves and Their In Vitro Hypoglycemic and Antioxidant Activities. Molecules 2023, 28, 3880. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, T.; Li, Y.; Liu, S.; Liu, Y.; Chen, D.; Qin, W.; Zhang, Q.; Lin, D.; Liu, Y.; et al. Ultrasound-Assisted Extraction of Tamarind Xyloglucan: An Effective Approach to Reduce the Viscosity and Improve the α-Amylase Inhibition of Xyloglucan. J. Sci. Food Agric. 2022, 103, 4047–4057. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Guo, Q.; Gao, X.; Ma, Q.; Xue, Z.; Ferri, N.; Zhang, M.; Chen, H. Identification of Ellagitannins in the Unripe Fruit of Rubus Chingii Hu and Evaluation of Its Potential Antidiabetic Activity. J. Agric. Food Chem. 2019, 67, 7025–7039. [Google Scholar] [CrossRef]
- Cuc, N.T.; Cuong, N.T.; Anh, L.T.; Yen, D.T.H.; Tai, B.H.; Thu Trang, D.; Yen, P.H.; Van Kiem, P.; Nam, N.H.; Van Minh, C.; et al. Dihydrostilbene Glycosides from Camellia Sasanqua and Their α-Glucosidase and α-Amylase Inhibitory Activities. Nat. Prod. Res. 2021, 35, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, J.; Ye, X.; Liu, D. In Vitro Inhibitory Effects of Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Leaves Proanthocyanidins on Pancreatic α-Amylase and Their Interaction. Bioorg. Chem. 2020, 101, 104029. [Google Scholar] [CrossRef]
- Dandekar, P.D.; Kotmale, A.S.; Chavan, S.R.; Kadlag, P.P.; Sawant, S.V.; Dhavale, D.D.; Ravikumar, A. Insights into the Inhibition Mechanism of Human Pancreatic α-Amylase, a Type 2 Diabetes Target, by Dehydrodieugenol B Isolated from Ocimum Tenuiflorum. ACS Omega 2021, 6, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.N.T.I.; Jayasinghe, S.; Attanayake, A.P.; Karunaratne, V.; Yaddehige, M.L.; Watkins, D.L. A New Dimeric Carbazole Alkaloid from Murraya koenigii (L.) Leaves with α-Amylase and α-Glucosidase Inhibitory Activities. Phytochem. Lett. 2022, 52, 87–91. [Google Scholar] [CrossRef]
- Naik, S.; Deora, N.; Pal, S.K.; Ahmed, M.Z.; Alqahtani, A.S.; Shukla, P.K.; Venkatraman, K.; Kumar, S. Purification, Biochemical Characterization, and DPP-IV and α-Amylase Inhibitory Activity of Berberine from Cardiospermum Halicacabum. J. Mol. Recognit. 2022, 35, e2983. [Google Scholar] [CrossRef]
- Thuy, N.T.K.; Phuong, P.T.; Hien, N.T.T.; Trang, D.T.; Van Huan, N.; Anh, P.T.L.; Tai, B.H.; Nhiem, N.X.; Hung, N.T.; Kiem, P. Van Pregnane Glycosides from the Leaves of Dregea Volubilis and Their α-Glucosidase and α-Amylase Inhibitory Activities. Nat. Prod. Res. 2021, 35, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Van Kiem, P.; Yen, D.T.H.; Van Hung, N.; Nhiem, N.X.; Tai, B.H.; Trang, D.T.; Yen, P.H.; Ngoc, T.M.; Van Minh, C.; Park, S.; et al. Five New Pregnane Glycosides from Gymnema Sylvestre and Their α-Glucosidase and α-Amylase Inhibitory Activities. Molecules 2020, 25, 2525. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, D.; Li, J.-B.; Zhang, X.; Zhou, N.; Zhang, W.-Y.; Lu, H. Prenylated Xanthones with α-Glucosidase and α-Amylase Inhibitory Effects from the Pericarp of Garcinia Mangostana. J. Asian Nat. Prod. Res. 2022, 24, 624–633. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.A.; Ahmed, S.; Abo-Haded, H. Garcixanthone D, a New Xanthone, and Other Xanthone Derivatives from Garcinia Mangostana Pericarps: Their α-Amylase Inhibitory Potential and Molecular Docking Studies. Starch/Staerke 2019, 71, 1800354. [Google Scholar] [CrossRef]
- Kawee-Ai, A.; Kim, A.T.; Kim, S.M. Inhibitory Activities of Microalgal Fucoxanthin against α-Amylase, α-Glucosidase, and Glucose Oxidase in 3T3-L1 Cells Linked to Type 2 Diabetes. J. Oceanol. Limnol. 2019, 37, 928–937. [Google Scholar] [CrossRef]
- Makinde, E.A.; Ovatlarnporn, C.; Sontimuang, C.; Herbette, G.; Olatunji, O.J. Chemical Constituents from the Aerial Part of Tiliacora Triandra (Colebr.) Diels and Their α-Glucosidase and α-Amylase Inhibitory Activity. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Ravi, L.; Girish, S.; D’Souza, S.R.; Anirudh Sreenivas, B.K.; Shree Kumari, G.R.; Archana, O.; Ajith Kumar, K.; Manjunathan, R. β-Sitosterol, a Phytocompound from Parthenium hysterophorus, Reveals Anti-Diabetic Properties through α-Amylase Inhibition: An i n-Silico and in-Vitro Analysis. J. Biomol. Struct. Dyn. 2023, 1–12. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The Inhibitory Effects of Flavonoids on α-Amylase and α-Glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as Potential Agents in the Management of Type 2 Diabetes through the Modulation of α-Amylase and α-Glucosidase Activity: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3137–3207. [Google Scholar] [CrossRef]
- Williams, L.K.; Li, C.; Withers, S.G.; Brayer, G.D. Order and Disorder: Differential Structural Impacts of Myricetin and Ethyl Caffeate on Human Amylase, an Antidiabetic Target. J. Med. Chem. 2012, 55, 10177–10186. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for Biosynthesis of Natural Product Drugs Using Engineered Microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.L.; Das, S. Phytochemicals and Their Effective Role in the Treatment of Diabetes Mellitus: A Short Review. Phytochem. Rev. 2018, 17, 1111–1128. [Google Scholar] [CrossRef]
- Etsassala, N.G.E.R.; Badmus, J.A.; Waryo, T.T.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A.; Iwuoha, E.I. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities of Novel Abietane Diterpenes from Salvia Africana-Lutea. Antioxidants 2019, 8, 421. [Google Scholar] [CrossRef] [Green Version]
- He, L.Y.; Li, Y.; Niu, S.Q.; Bai, J.; Liu, S.J.; Guo, J.L. Polysaccharides from Natural Resource: Ameliorate Type 2 Diabetes Mellitus via Regulation of Oxidative Stress Network. Front. Pharmacol. 2023, 14, 1184572. [Google Scholar] [CrossRef]
- Wang, P.-C.; Zhao, S.; Yang, B.-Y.; Wang, Q.-H.; Kuang, H.-X. Anti-Diabetic Polysaccharides from Natural Sources: A Review. Carbohydr. Polym. 2016, 148, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms Underlying the Effect of Polysaccharides in the Treatment of Type 2 Diabetes: A Review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Jia, X.; Wang, N.; Kang, J.; Hu, X.; Goff, H.D.; Cui, S.W.; Ding, H.; Guo, Q. Therapeutic Potential of Non-Starch Polysaccharides on Type 2 Diabetes: From Hypoglycemic Mechanism to Clinical Trials. Crit. Rev. Food Sci. Nutr. 2022, 1–34. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent Advances in Polysaccharides from Lentinus Edodes (Berk.): Isolation, Structures and Bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef]
- Cao, C.; Li, C.; Chen, Q.; Huang, Q.; Pérez, M.E.M.; Fu, X. Physicochemical Characterization, Potential Antioxidant and Hypoglycemic Activity of Polysaccharide from Sargassum Pallidum. Int. J. Biol. Macromol. 2019, 139, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhao, Q.; Zhao, B. Physicochemical Properties, Structural Characterization and Biological Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Int. J. Biol. Macromol. 2021, 193, 1635–1644. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhong, R.F.; Chen, J.; Luo, Z.G. Structural Characterization, Anticancer, Hypoglycemia and Immune Activities of Polysaccharides from Russula Virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Reports 2019, 24, e00370. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phyther. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Marsh, K.J.; Wallis, I.R.; Kulheim, C.; Clark, R.; Nicolle, D.; Foley, W.J.; Salminen, J.P. New Approaches to Tannin Analysis of Leaves Can Be Used to Explain in Vitro Biological Activities Associated with Herbivore Defence. New Phytol. 2020, 225, 488–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajebli, M.; Eddouks, M. The Promising Role of Plant Tannins as Bioactive Antidiabetic Agents. Curr. Med. Chem. 2018, 26, 4852–4884. [Google Scholar] [CrossRef] [PubMed]








| Name of Plants | Used Extract/Fraction/Parts | IC50 | IC50 (Control) (Acarbose) | Ref. |
|---|---|---|---|---|
| Argania spinosa | Roasted seeds oil | 2.17 ± 0.24 mg/mL | 0.41 ± 0.015 mg/mL | [63] |
| Unroasted seeds oil | 0.78 ± 0.16 mg/mL | |||
| Melicope glabra (Blume) T. G. Hartley | Chloroform extracts (leaves) | 303.64 ± 10.10 μg/mL | 188.6 ± 14.31 μg/mL | [64] |
| Aloe megalacantha Baker | Leaf latex | 74.76 ± 1.98 μg/mL | 16.49 ± 1.91 μg/mL | [65] |
| Aloe monticola Reynolds | 78.10 ± 1.88 μg/mL | 16.49 ± 1.91 μg/mL | ||
| Hedychium coronarium Koen | Ethyl acetate fraction (rhizomes) | 58.15 ± 1.23 μg/mL | - | [66] |
| Cichorium endivia | Hydrophilic fraction (extracted from leaves with ethanol/water) | 9.96 μg/mL | 10.00 μg/mL | [67] |
| Brown rice | Brown rice (BR) extract | 48.96 ± 0.34% | 54.14 ± 0.35% | [68] |
| BR/Vanillin | 99.32 ± 1.18% | 86.48 ± 0.71% | ||
| BR/Vanillyl alcohol | 96.55 ± 0.12% | |||
| Biyun no.7 (Kidney bean) | Aqueous extract | 1.659 ± 0.050 U/g DW | - | [69] |
| Rhus coriaria L. | Ethyl acetate sub-extract (leaves) | 20.810 ± 0.747 μg/mL | 26.993 ± 0.797 μg/mL | [70] |
| Melicope latifolia | Chloroform extract (bark) | 1464.32 μg/mL | [71] | |
| Arachis hypogaea | Ethanol extract (peanut seeds) | 0.61 μg/mL | 0.32 μg/mL | [72] |
| Backhousia citriodora | Essential oil | 0.49 mg/μL | - | [73] |
| Rosmarinus officinalis | Essential oil of rosemary plus | 0.45 mg/μL | ||
| Mentha piperita | Essential oil (leaf) | 0.41 mg/μL | ||
| Origanum vulgare | Essential oil (leaf-phenol type) | 0.41 mg/μL | ||
| Quercus variabilis Blume | Free polyphenol extract | 5.25 ± 0.57 mg/mL | 0.24 mg/mL | [74] |
| Bound polyphenol extract | 1.37 ± 0.11 mg/mL | |||
| Centaurea pterocaula Trautv | Essential oil (aerial part) | 79.66 ± 0.43 µg/mL | 11.6 ± 0.18 µg/mL | [75] |
| Anchusa officinalis | Crude extract | 954.16 ± 7.46 µg/mL | 17.68 ± 1.24 µg/mL | [76] |
| Melilotus officinalis | Crude extract | 1.32 ± 0.08 µg/mL | ||
| Clausena indica | Hexane extract | 1.37 ± 0.01 mg/mL | 0.07 ± 0.00 mg/mL | [77] |
| Ethyl acetate extract | 8.56 ± 0.24 mg/mL | |||
| Prosopis cineraria (L.) | n-Butanol fraction (pods) | 22.01 ± 0.92 μg/mL | 39.26 ± 2.19 μg/mL | [78] |
| Ethyl acetate fraction | 28.23 ± 1.06 μg/mL | |||
| Senna auriculata (L.) Roxb. | Methanolic extract (leaves) | 49.45 μg/mL | - | [79] |
| Terfezia claveryi | Methanol extract | 38.7 μg/mL | 45.3 μg/mL | [80] |
| Rhododendron arboreum Sm. | Methanol extract | 51.1% | - | [81] |
| Phragmites karka (Retz.) | Dichloromethane fraction (aerial part) | 2.05 mg/mL | - - | [82] |
| n-Hexane fraction (aerial part) | 2.08 mg/mL | |||
| Morus nigra | Hexane fraction (leaves) | 13.05 mg/mL | 0 0.21 mg/mL | [83] |
| Salaria basilisca | Protein hydrolysates (peptide fraction F1) | 71 μg/mL | 14 μg/mL | [84] |
| Leucaena leucocephala (Lam.) De Wit | Ethanol extract (leaves) | 288.01 μg/mL | 252.59 μg/mL | [85] |
| Bergenia pacumbis | Methanol extract | 14.03 ± 0.04 μg/mL | 20.12 ± 0.12 μg/mL | [86] |
| Nonea obtusifolia (Wild.) DC. | Acetone extract | 25.7 ± 0.08 μg/mL | 28.18 ± 1.22 μg/mL | [87] |
| Morus alba Linn | Leaves | 74.76 ± 6.76 μg/mL | 35.34 ± 4.87 μg/mL | [88] |
| Phylanthus emblica L. | Methanolic extract (leaves) | 98.37 ± 1.09% | [89] | |
| Catunaregam spinosa | Dichloromethane fraction (bark) | 77.17 ± 1.75 μg/mL | 6.34 ± 0.07 μg/mL | [90] |
| Chenopodium album L. | Flavonoid fraction (aerial part) | 122.18 ± 1.15 μg/mL | 812.83 ± 1.07 μg/mL | [91] |
| Solanum virginianum | Aqueous extract (fruits) | 54.12 ± 0.44–86.80 ± 0.27% | 58.36 ± 0.30–88.24 ± 0.16% | [92] |
| Ethanolic extract (fruits) | 23.07 ± 0.47–81.61 ± 0.43% | |||
| Eucalyptus globulus | Ethanol extract (Hexane defatted) | 23.6 ± 1.2 μg/mL | 5.2 ± 1.3 μg/mL | [93] |
| Ethanol extract (non-defatted) | 14.8 ± 1.2 μg/mL | |||
| Maesobotrya dusenii Hutch. | Crude methanol extract | 24 μg/mL | 28 μg/mL | [94] |
| Veronica biloba | Aqueous extract | 110.25 μg/mL | 138.79 μg/mL | [95] |
| Ethyl acetate extract | 121.09 μg/mL | |||
| Dichloromethane extract | 123.68 μg/mL | |||
| Salvia lavandulifolia Vahl | Aqueous extract | 0.99 ± 0.00 mg/mL | 0.52 ± 0.01 mg/mL | [96] |
| Moringa oleifera | Methanolic crude extract (leaves) | 65.6 ± 4.93% | - | [97] |
| Ziziphus mucronata | Acetone extract | 0.62 mg/mL | 0.42 mg/mL | [98] |
| Englerophytum magalismontanum | Methanol fraction (leaves) | 10.76 ± 1.33 µg/mL | 1.24 ± 1.64 µg/mL | [99] |
| Achyranthes aspera | Crude extract | 97.60 ± 1.11 μg/mL | 68.13 ± 0.46 μg/mL | [100] |
| Catharanthus roseus | 94.05 ± 1.18 μg/mL | |||
| Pterocarpus marsupium | Methanolic extract | 158.663 ± 10.986 μg/mL | 56.060 ± 4.465 μg/mL | [101] |
| Rubus corchorifolius L. | 70% ethanolic extract (leaf tea) | 1.26 ± 0.03 mg/mL | 5.12 ± 0.42 mg/mL | [102] |
| 70% methanolic extract (leaf tea) | 1.47 ± 0.05 mg/mL | |||
| Aqueous extract (leaf tea) | 4.39 ± 0.17 mg/mL | |||
| Sterculia nobilis Smith | Ethyl acetate fraction (pericarp) | 13.550 ± 0.230 μg/mL | 19.45 ± 0.26 μg/mL | [103] |
| Plant Extracts | Dosage of Plant Extract Used | Experimental Animals | Types of Diabetes Induction | Administration Route | Diagnostic Criteria | Inference | Ref. |
|---|---|---|---|---|---|---|---|
| Oxalis pes-caprae Methanolic extract | 150 mg/kg BW | Swiss albino mice | Alloxan-induced diabetes | Intraperitoneal injection | Fasting blood glucose (FBG) level, body weight | Hypoglycemic | [122] |
| Cardamine hirsuta Linn Hydro-methanolic extract | 125, 250, and 500 mg/kg BW | Male Sprague Dawley (SD) rats | High-fat diet (HFD), Streptozotocin (STZ)-induced diabetes | Oral and intraperitoneal injection | FBG level | Hypoglycemic, dose-dependent | [123] |
| Trachinotus ovatus Protein hydrolysates | 100, 500, and 1000 mg/kg BW | Male Kunming mice | STZ-induced diabetes | Intraperitoneal injection | FBG level | Hypoglycemic, dose-dependent | [124] |
| Sorbaria tomentosa Lindl. Rehder Methanolic extract | 150 and 300 mg/kg BW | Rats | Alloxan-induced diabetes | Intraperitoneal injection | FBG level | Hypoglycemic | [125] |
| Chenopodium album L. Flavonoid fraction | 500 mg/kg BW | SD rats | HFD-STZ-induced diabetes | Oral and intraperitoneal injection | Glucose, cholesterol, and triglyceride levels | Hypoglycemic | [91] |
| Terfezia claveryi Methanolic extract | 200 mg/kg BW | Male Wistar albino rats | STZ-induced diabetes | Intraperitoneal injection | FBG level | Hypoglycemic time-dependent | [80] |
| Salvia lavandulifolia Vahl Aqueous extract | 400 mg/kg BW | Normal rats | D-glucose, 2 g/kg | Oral | Oral glucose tolerance test (OGTT) Blood glucose level | Hypoglycemic | [96] |
| Artemisia absinthium L. Aqueous extract | 200 mg/kg BW | Wistar rats | Alloxan-induced diabetes | Oral | Postprandial blood glucose (PBG) level | Hypoglycemic | [126] |
| Ammodaucus leucotrichus Coss. and Durieu Aqueous extract | 150 mg/kg BW | Wistar albino rats | Alloxan-induced diabetes | Oral | OGTT | Hypoglycemic | [127] |
| Porphyra spp. Polysaccharides | 100 mg/kg BW | Rats | - | Oral | PBG level | Hypoglycemic | [128] |
| Prosopis cineraria Ethyl acetate fraction/n-Butanol fraction | 250, 500, 1000 and 2000 mg/kg BW | Swiss albino mice | Sucrose tolerance test (OSTT) | Oral | Serum glucose concentration | Hypoglycemic | [78] |
| Allium sativum L. Polysaccharides | 1.25, 2.5, and 5 g/kg BW | Male Kunming mice | STZ-induced diabetes | Intraperitoneal injection | OGTT, FBG | Hypoglycemic | [129] |
| Class of Compounds | Bioactive Compound | Source (Plant’s Name) | IC50 | IC50 (Control) (Acarbose) | Ref. |
|---|---|---|---|---|---|
| Flavonoids | 5-hydroxy-2-(4-methoxy-3-((E)-3-methylbut-1-enyl)-5-(3-methylbut-3-enyl)phenyl)chroman-4-one | Andrographis echioides | 3.357 µg/mL | [134] | |
| Luteolin | Taraxacum mongolicum | 42.33 ± 0.82 μg/mL | - | [135] | |
| Isoquercitrin | Melilotus officinalis | 9.65 ± 0.43 μg/mL | 17.68 ± 1.24 μg/mL | [76] | |
| Epicatechin gallate | Euryale ferox (seed coat) | 0.92 mg/mL | 1.08 mg/mL | [136] | |
| Puerarin | Agave americana L. | 3.87 μM | [137] | ||
| Tricetin | Punica granatum | 0.43 ± 0.12 mg/mL | 0.038 ± 0.017 mg/mL | [138] | |
| Flavonoid glucoside | Tricetin 4′-O-β-glucopyranoside | 1.17 ± 0.32 mg/mL | |||
| Rutin | Melilotus officinalis | 11.42 ± 0.62 μg/mL | 17.68 ± 1.24 μg/mL | [76] | |
| quercetagetin-7-O-β-D-glucopyranoside | Tagetes minuta L. | 7.8 µM | 7.1 µM | [139] | |
| luteolin-7-O-α-L-rhamnoside | Phylanthus emblica L. | 89.79% | - | [89] | |
| hypolaetin 8-O-β-D-galactopyranoside | Thymelaea tartonraira (leaves) | 46.49 ± 2.32 μg/mL | 0.44 ± 0.022 μg/mL | [140] | |
| Ent-kaurane diterpenoids | Wedtriloside A | Wedelia trilobata (leaves) | 112.20 ± 2.87 μg/mL | - | [141] |
| Wedtriloside B | 87.10 ± 1.89 μg/mL | ||||
| Abietane diterpene | Carnosol | Salvia aurita (aerial part) | 19.8 ± 1.4 µg/mL | 10.2 ± 0.6 µg/mL | [142] |
| 12-methoxycarnosic acid | 16.2 ± 0.3 µg/mL | ||||
| Pentacyclic triterpenoid | Oleanolic acid | Xylopia aethiopica (Dunal) A. Rich. (fruit) | 89.02 ± 1.12 µM | - | [143] |
| Triterpenoid | Glochidon | Phyllanthus debilis | 38.15 ± 1.40 μM | 33.68 ± 3.12 μM | [144] |
| Triterpenoid saponin | Ligularoside A | Passiflora ligularis Juss (leaves) | 409.8 ± 11.4 μM | 234.1 ± 15.9 μM | [145] |
| Phenylpropanoids | Jionoside D | Clerodendrum infortunatum L. | 3.4 ± 0.2 μM | 5.9 ± 0.1 μM | [146] |
| Polysaccharides | WSRP-2a | Rosa setate × Rosa rugosa (waste biomass) | 3.41 mg/mL | 0.57 mg/mL | [147] |
| WSRP-2b | 1.72 mg/mL | ||||
| PD-1 | Porphyra spp. | 12.72 mg/mL | [128] | ||
| SGP-1-1 | Siraitia grosvenorii | 61.73% at 1 mg/mL | [148] | ||
| LLP50 (polysaccharides fraction) | Lycium barbarum (leaves) | 1.659 mg/mL | 0.0002 mg/mL | [149] | |
| Xyloglucan | Tamarindus indica L | 72.49 ± 0.84% | 92.49 ± 1.97% | [150] | |
| Phenolic acid and its derivatives | Ellagic acid | Quercus variabilis Blume | 0.19 ± 0.02 μg/mL | 0.24 μg/mL | [74] |
| Rosmaric acid | Anchusa officinalis | 0.92 ± 0.07 μg/mL | 17.68 ± 1.24 μg/mL | [76] | |
| Chlorogenic acid | 1.84 ± 0.05 μg/mL | ||||
| p-Coumaric acid | Agave americana L. | 10.16 μM | - | [137] | |
| Ellagitannins | Chingiitannin A | Rubus chingii Hu (unripe fruit/n-BuOH fraction) | 4.52 ± 0.30 μM | 35.71 ± 4.93 μM | [151] |
| Lambertianin A | 10.32 ± 0.10 μM | ||||
| Sanguiin H-6 | 11.00 ± 0.21 μM | ||||
| Gallotanins | 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose | Rhus coriaria (leaves) | 6.32 ± 0.18 μM | 10.69 ± 0.50 μM | [70] |
| Dihydrostilbene glycosides | Sasastilboside A | Camellia sasanqua Thunb (leaves) | 53.7 ± 1.6 μM | - | [152] |
| Proanthocyanidins | Chinese bayberry leaves proanthocyanidins | Myrica rubra Sieb. et Zucc. (leaves) | 3.075 ± 0.073 μg/mL | - | [153] |
| Phenolic | Dehydrodieugenol B | Ocimum tenuiflorum | 29.6 μM | 13.85 μM | [154] |
| Coumarin | Halfordin | Melicope latifolia | 197.53 µM | 282.39 ± 8.14 µM | [71] |
| Alkaloids | 3,3′,5,5′,8-pentamethyl-3,3′-bis(4-methylpent-3-en-1-yl)-3,3′,11,11′-tetrahydro-10,10′-bipyrano[3,2-a]carbazole | Murraya koenigii (L.) | 30.32 ± 0.34 ppm | - | [155] |
| Berberine | Cardiospermum halicacabum | 72% at 10 μg/mL | [156] | ||
| Pregnane glycosides | Drevoluoside Q | Dregea volubilis (leaves) | 51.3 ± 2.1 µM | 36.3 ± 0.5 µM | [157] |
| Gymsyloside B | Gymnema sylvestre (leaves) | 175.8 ± 2.3 µM | 72.4 ± 0.8 µM | [158] | |
| Gymsyloside C | 162.2 ± 2.7 µM | ||||
| Gymsyloside D | 113.0 ± 0.7 µM | ||||
| Prenylated xanthones | Mangoxanthone A | Garcinia mangostana (pericarp) | 22.74 ± 2.07 µM | - | [159] |
| Xanthone | Garcixanthone D | Garcinia mangostana (pericarp) | 93.8% | 96.4% | [160] |
| Garcinone E | 85.6% | ||||
| Xanthophyll | Fucoxanthin | Phaeodactylum tricornutum | 28.38 ± 0.67 mmol/L | 25.01 ± 1.38 mmol/L | [161] |
| Fatty acid | 5,7-dihydroxy-6-oxoheptadecanoic acid | Tiliacora triandra | 26.27 ± 1.11 μM. | 177.65 ± 0.88 μM | [162] |
| Phytosterols | β-Sitosterol | Parthenium hysterophorus (leaves) | 42.30% (at 400 µg/mL) | - | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashtoh, H.; Baek, K.-H. New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants 2023, 12, 2944. https://doi.org/10.3390/plants12162944
Kashtoh H, Baek K-H. New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants. 2023; 12(16):2944. https://doi.org/10.3390/plants12162944
Chicago/Turabian StyleKashtoh, Hamdy, and Kwang-Hyun Baek. 2023. "New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects" Plants 12, no. 16: 2944. https://doi.org/10.3390/plants12162944
APA StyleKashtoh, H., & Baek, K.-H. (2023). New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants, 12(16), 2944. https://doi.org/10.3390/plants12162944








