Exogenous Sodium Nitroprusside Affects the Redox System of Wheat Roots Differentially Regulating the Activity of Antioxidant Enzymes under Short-Time Osmotic Stress
Abstract
:1. Introduction
2. Results
2.1. O2•− Production and SOD Activity in Roots of Wheat Seedlings Pretreated with SNP and Subjected to Osmotic Stress for 4 h
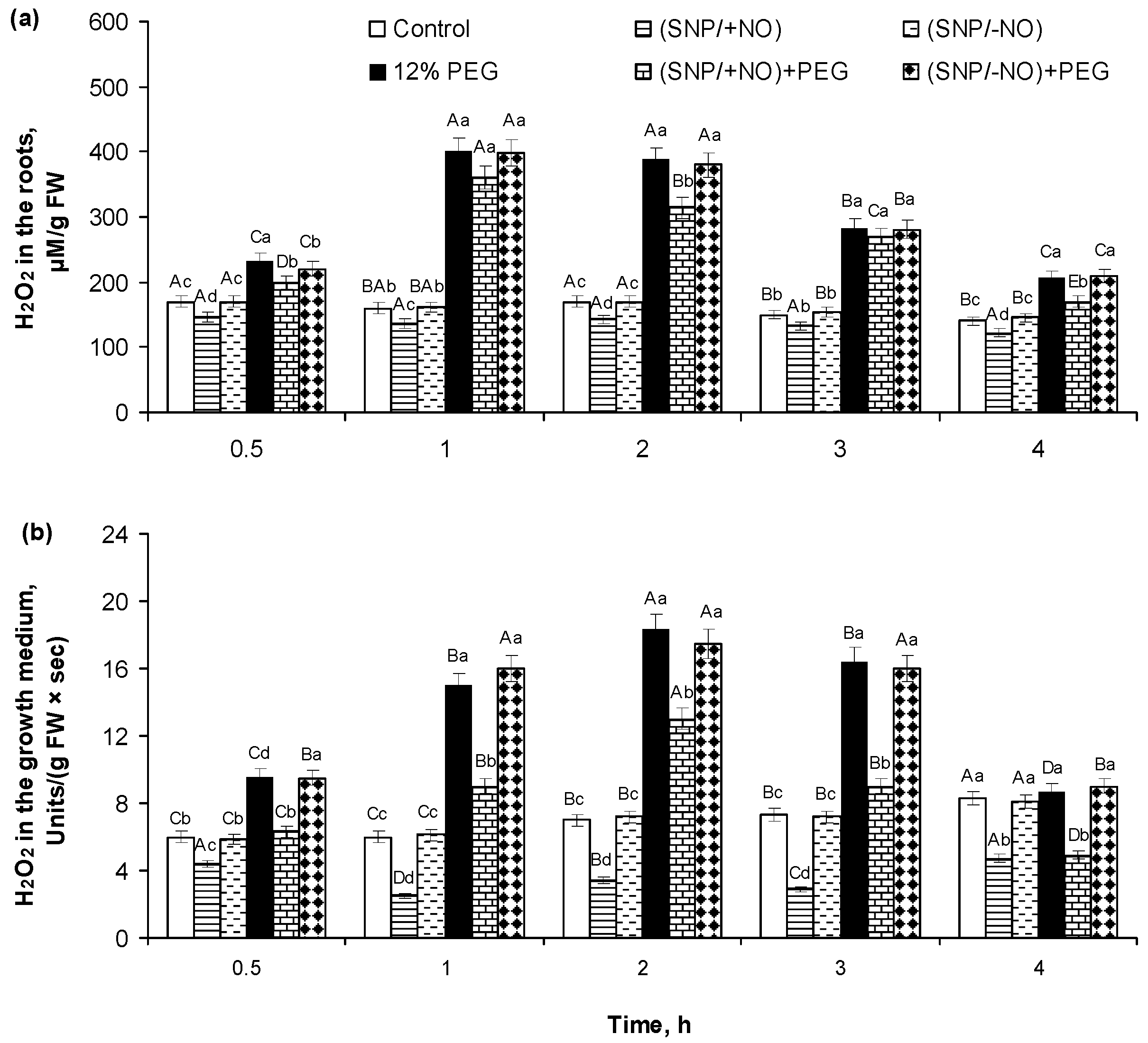
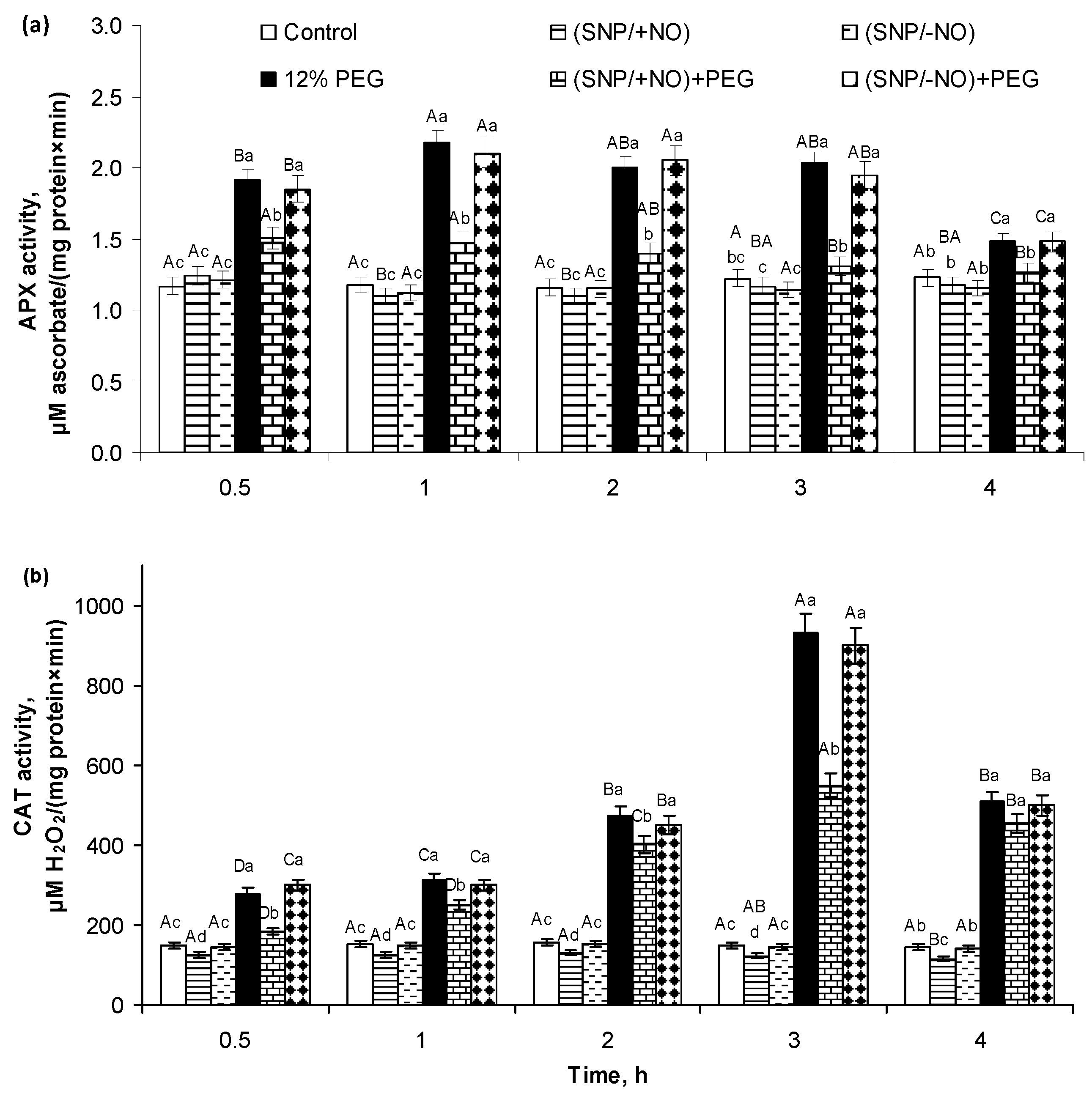
2.2. H2O2 Production and Activity of APX and CAT in Roots of Wheat Seedlings Pretreated with SNP and Subjected to Osmotic Stress for 4 h
2.3. ROS Content and Antioxidant Enzymes Activity after 24 h of 12% PEG Exposure
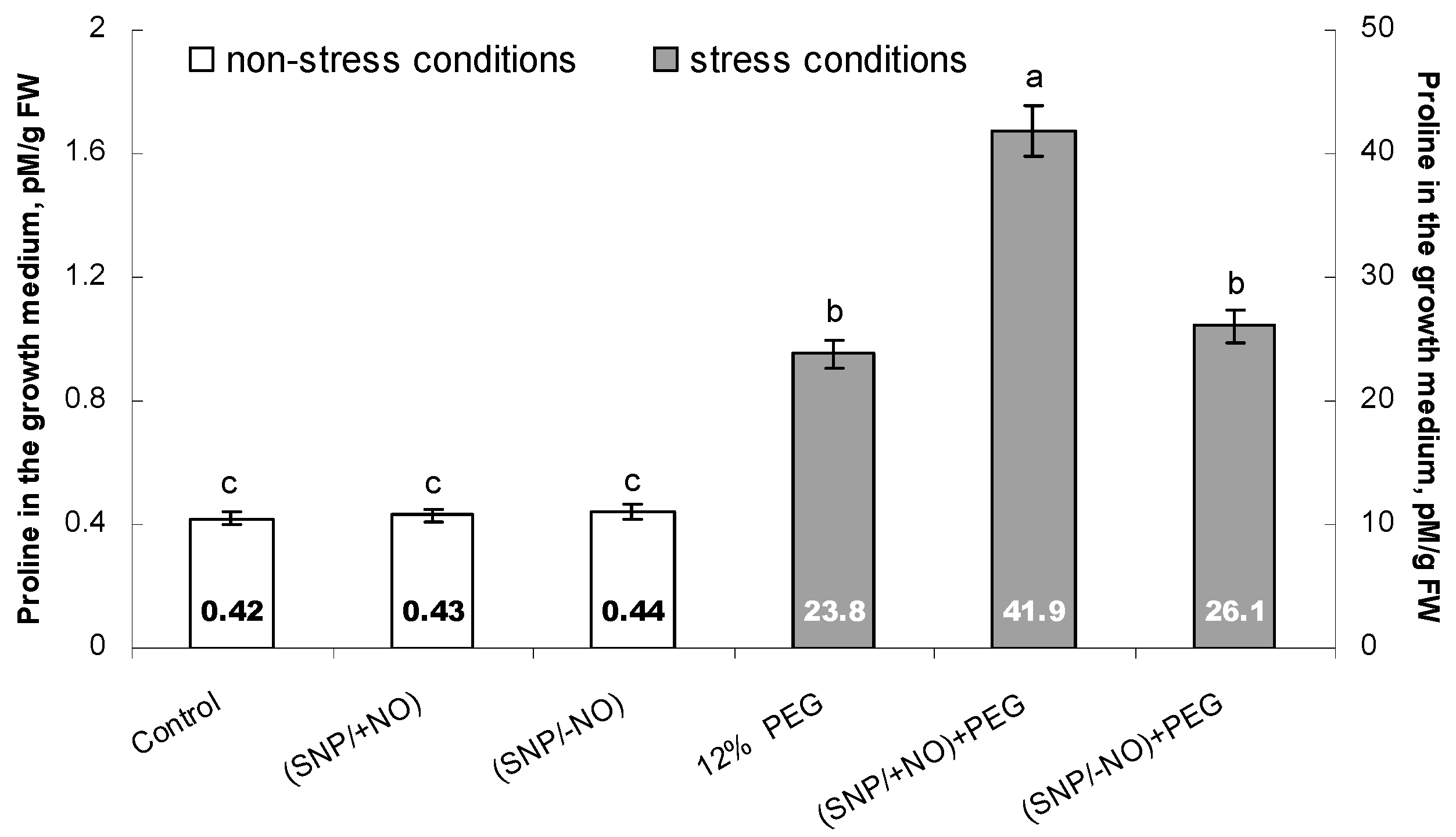
2.4. Proline Accumulation in the Growth Medium
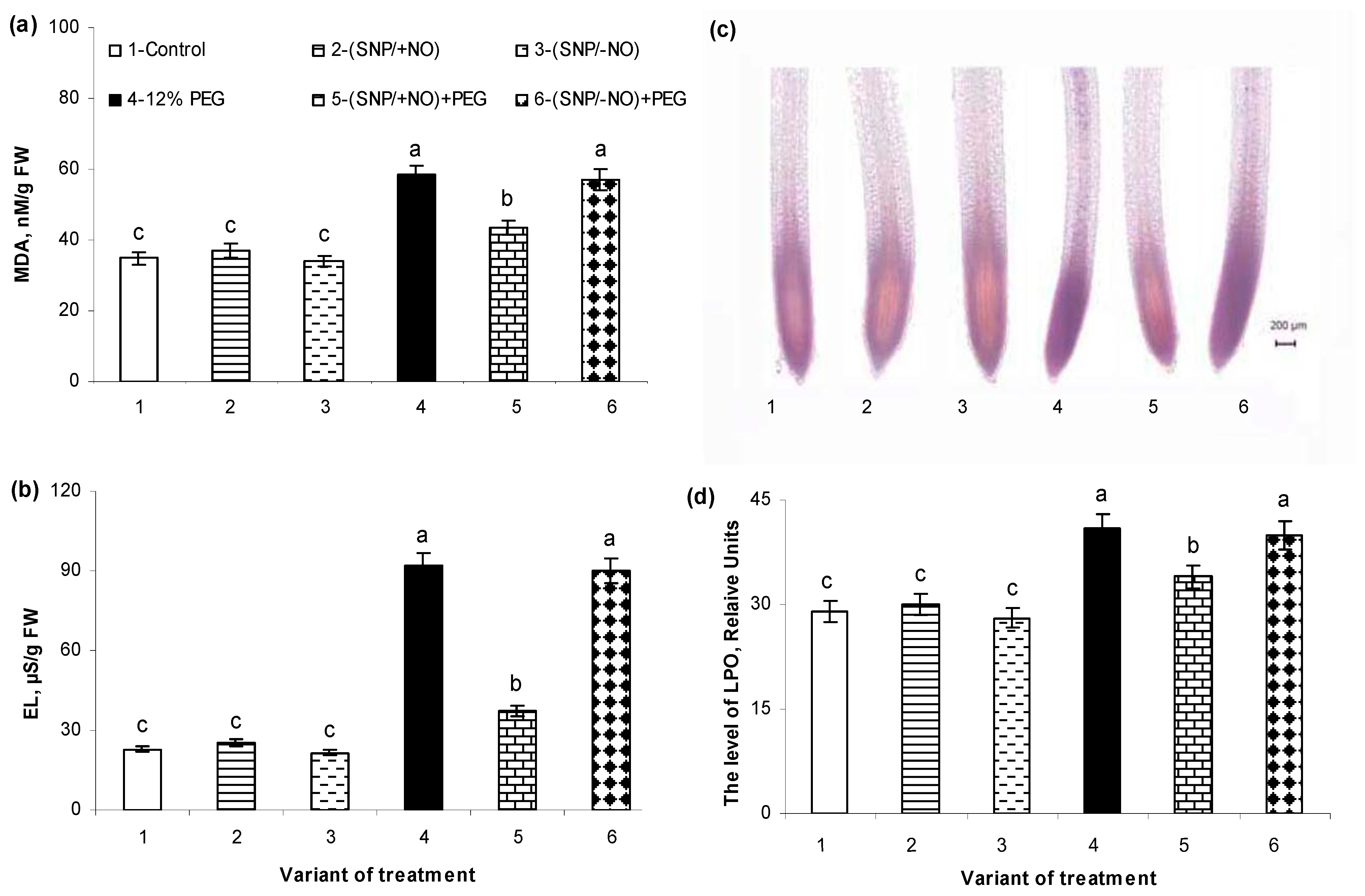
2.5. The Levels of Malondialdehyde Accumulation and Electrolytes Leakage
2.6. Histochemical Detection of Lipid Peroxidation
2.7. The Percent of Cell Death and MI of Meristematic Cells of Wheat Roots
2.8. Correlation Matrices
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Assay of Antioxidant Enzymes
- OD—optical density in experimental samples;
- ODcontrol—optical density of control samples;
- T—time of reaction, min;
- X—terminal dilution of extraction solution in the well;
- L—layer thickness, cm;
- C—protein content in the probe, mg.
4.3. Determination of Superoxide Radical in the Growth Medium
4.4. Estimation of O2•− Content in the Roots of Wheat Seedlings
4.5. Extracellular H2O2 Content
4.6. Estimation of H2O2 Content in the Roots of Wheat Seedlings
4.7. Malondialdehyde (MDA) Accumulation
4.8. Estimation of Electrolyte Leakage (EL)
4.9. Proline Accumulation in the Growth Medium
4.10. Analysis of Root Cell Death
4.11. Histochemical Detection of Lipid Peroxidation
4.12. Mitotic Index (MI) Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Rodrigues, F.; Sousa, B.; Pinto, E.; Ferreira, I.M.P.L.V.O.; Pereira, R.; Fidalgo, F. Foliar Application of Sodium Nitroprusside Boosts Solanum lycopersicum L. Tolerance to Glyphosate by Preventing Redox Disorders and Stimulating Herbicide Detoxification Pathways. Plants 2021, 10, 1862. [Google Scholar] [CrossRef] [PubMed]
- Skliros, D.; Kalloniati, C.; Karalias, G.; Skaracis, G.N.; Rennenberg, H.; Flemetakis, E. Global Metabolomics Analysis Reveals Distinctive Tolerance Mechanisms in Different Plant Organs of Lentil (Lens culinaris) upon Salinity Stress. Plant Soil 2018, 429, 451–468. [Google Scholar] [CrossRef]
- Signorelli, S.; Corpas, F.J.; Borsani, O.; Barroso, J.B.; Monza, J. Water Stress Induces a Differential and Spatially Distributed Nitro-oxidative Stress Response in Roots and Leaves of Lotus japonicas. Plant Sci. 2013, 201–202, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric Oxide Stimulates Antioxidant System and Osmotic Adjustment in Soybean Under Drought Stress. J. Soil Sci. Plant Nutr. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Vainer, A.A.; Yastreb, T.O. Proline: Physiological Functions and Regulation of Content in Plants Under Stress Conditions. Visn. Khark. Nats. Agrar. Univ. Ser. Biol. 2014, 2, 6–22. [Google Scholar]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Karpets, Y.V. Reactive Oxygen Species and Stress Signaling in Plants. Ukr. Biochem. J. 2014, 86, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Allagulova, C.R.; Lubyanova, A.R.; Avalbaev, A.M. Multiple Ways of Nitric Oxide Production in Plants and Its Functional Activity under Abiotic Stress Conditions. Int. J. Mol. Sci. 2023, 24, 11637. [Google Scholar] [CrossRef]
- Del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant Superoxide Dismutases: Function Under Abiotic Stress Conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 13–26. [Google Scholar] [CrossRef]
- Paul, S.; Roychoudhury, A. Regulation of Physiological Aspects in Plants by Hydrogen Sulfide and Nitric Oxide Under Challenging Environment. Physiol. Plant. 2020, 168, 374–393. [Google Scholar] [CrossRef]
- Roy, S. Role of Nitric Oxide as a Double Edged Sword in Root Growth and Development. In Rhizobiology: Molecular Physiology of Plant Roots. Signaling and Communication in Plants; Mukherjee, S., Baluška, F., Eds.; Springer International Publishing AG: Cham, Switzerland, 2021; pp. 167–193. [Google Scholar] [CrossRef]
- Corpas, F.J.; Palma, J.M. Functions of NO and H2S Signal Molecules Against Plant Abiotic Stress. In Plant Abiotic Stress Signaling. Methods in Molecular Biology; Couée, I., Ed.; Humana: New York, NY, USA, 2023; Volume 2642, pp. 97–109. [Google Scholar] [CrossRef]
- Allagulova, C.; Avalbaev, A.; Lubyanova, A.; Plotnikov, A.; Yuldashev, R.; Lastochkina, O. Nitric Oxide (NO) Improves Wheat Growth under Dehydration Conditions by Regulating Phytohormone Levels and Induction of the Expression of the TADHN Dehydrin Gene. Plants 2023, 12, 4051. [Google Scholar] [CrossRef]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric Oxide Imbalance Provokes a Nitrosative Response in Plants Under Abiotic Stress. Plant Sci. 2011, 181, 604. [Google Scholar] [CrossRef]
- Yang, H.; Mu, J.; Chen, L.; Feng, J.; Hu, J.; Li, L.; Zhou, J.M.; Zuo, J. S-Nitrosylation Positively Regulates Ascorbate Peroxidase Activity During Plant Stress Responses. Plant Physiol. 2015, 167, 1604–1615. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Correa-Aragunde, C.; Foresi, N.; Lamattina, L. Nitric Oxide is a Ubiquitous Signal for Maintaining Redox Balance in Plant Cells: Regulation of Ascorbate Peroxidase as a Case Study. J. Exp. Bot. 2015, 66, 2913–2921. [Google Scholar] [CrossRef]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant Catalases as NO and H2S Targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Lubyanova, A.R.; Bezrukova, M.V.; Shakirova, F.M. Involvement of Nitric Oxide in Methyl Jasmonate-Mediated Regulation of Water Metabolism in Wheat Plants under Drought Stress. Stresses 2022, 2, 477–492. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Lanteri, M.L.; Lombardo, M.C.; Lamattina, L. Nitric Oxide Mediates the Indole Acetic Acid Induction Activation of a Mitogen-Activated Protein Kinase Cascade Involved in Adventitious Root Development. Plant Physiol. 2004, 135, 279. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric Oxide Plays a Central Role in Determining Lateral Root Development in Tomato. Planta 2004, 218, 900. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Functions of Nitric Oxide (NO) in Roots during Development and under Adverse Stress Conditions. Plants 2015, 4, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Wimalasekera, R.; Scherer, G.F.E. Nitric Oxide (NO) and Lateral Root Development in Plants Under Stress. In Nitric Oxide in Plant Biology: An Ancient Molecule with Emerging Roles; Singh, V.P., Singh, S., Sandalio, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 319–329. [Google Scholar] [CrossRef]
- Maslennikova, D.R.; Allagulova, C.R.; Fedorova, K.A.; Plotnikov, A.A.; Avalbaev, A.M.; Shakirova, F.M. Cytokinins Contribute to Realization of Nitric Oxide Growth-Stimulating and Protective Effects on Wheat Plants. Russ. J. Plant Physiol. 2017, 64, 665–671. [Google Scholar] [CrossRef]
- Groß, F.; Durner, J.; Gaupels, F. Nitric Oxide, Antioxidants and Prooxidants in Plant Defence Responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar] [CrossRef] [PubMed]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Ejaz, S.; Ahmad, K.S.; Tauseef, S.; Farid, G.; Khalid, I.; Mehmood, K. Nitric Oxide Regulates Water Status and Associated Enzymatic Pathways to Inhibit Nutrients Imbalance in Maize (Zea mays L.) Under Drought Stress. Plant Physiol. Biochem. 2020, 155, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alam, P.; Ahmad, P. Nitric Oxide and Hydrogen Sulfide Work Together to Improve Tolerance to Salinity Stress in Wheat Plants by Upraising the AsA-GSH Cycle. Plant Physiol. Biochem. 2023, 194, 651–663. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive Oxygen Species in Plant Development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.S.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric Oxide-Mediated Regulation of Oxidative Stress in Plants Under Metal Stress: A Review. Physiol. Plant. 2020, 168, 318–344. [Google Scholar] [CrossRef]
- Iqbal, N.; Sehar, Z.; Fatma, M.; Umar, S.; Sofo, A.; Khan, N.A. Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants. Antioxidants 2022, 11, 372. [Google Scholar] [CrossRef]
- Bari, M.A.; Akther, M.S.; Reza, M.A.; Kabir, A.H. Cadmium Tolerance is Associated with the Root-Driven Coordination of Cadmium Sequestration, Iron Regulation, and ROS Scavenging in Rice. Plant Physiol. Biochem. 2019, 136, 22–33. [Google Scholar] [CrossRef]
- Fichman, Y.; Mittler, R. A Systemic Whole-Plant Change in Redox Levels Accompanies the Rapid Systemic Response to Wounding. Plant Physiol. 2021, 186, 4–8. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Kokorev, A.I. Antioxidant System and Plant Resistance to Water Deficit. Fiziol. Rast. Genet. 2019, 51, 28–54. (In Russian) [Google Scholar] [CrossRef]
- Cao, X.; Zhu, C.; Zhong, C.; Zhang, J.; Wu, L.; Jin, Q.; Ma, Q. Nitric Oxide Synthase-Mediated Early Nitric Oxide Burst Alleviates Water Stress-Induced Oxidative Damage in Ammonium-Supplied Rice Roots. BMC Plant Biol. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, S.; Xuan, Y.; Sun, M.; Zhao, L. Protective Effect of Nitric Oxide Against Oxidative Damage in Arabidopsis Leaves Under Ultraviolet-B Irradiation. J. Plant Biol. 2009, 52, 135–140. [Google Scholar] [CrossRef]
- Keisham, M.; Jain, P.; Singh, N.; von Toerne, C.; Bhatla, S.C.; Lindermayr, C. Deciphering The Nitric Oxide, Cyanide and Iron-Mediated Actions of Sodium Nitroprusside in Cotyledons of Salt Stressed Sunflower Seedlings. Nitric Oxide 2019, 88, 10–26. [Google Scholar] [CrossRef]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A Forty Year Journey: The Generation and Roles of NO in Plants. Nitric Oxide 2019, 93, 53. [Google Scholar] [CrossRef]
- Gouvea, C.M.C.P.; Souza, J.F.; Magalhaes, A.C.N.; Martins, I.S. NO•–Releasing Substances That Induce Growth Elongation in Maize Root Segments. Plant Growth Regul. 1997, 21, 183–187. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Graziano, M.; Chevalier, C.; Lamattina, L. Nitric Oxide Modulates the Expression of Cell Cycle Regulatory Genes During Lateral Root Formation in Ttomato. J. Exp. Bot. 2006, 57, 581–588. [Google Scholar] [CrossRef]
- Batista, P.F.; Costa, A.C.; Müller, C.; de Oliveira Silva-Filho, R.; da Silva, F.B.; Merchant, A.; Mendes, G.C.; Nascimento, K.J.T. Nitric Oxide Mitigates the Effect of Water Deficit in Crambe abyssinica. Plant Physiol. Biochem. 2018, 129, 310–322. [Google Scholar] [CrossRef]
- Holzmeister, C.; Gaupels, F.; Geerlof, A.; Sarioglu, H.; Sattler, M.; Durner, J.; Lindermayr, C. Differential Inhibition of Arabidopsis Superoxide Dismutases by Peroxynitrite-Mediated Tyrosine Nitration. J. Exp. Bot. 2015, 66, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, X.; Zhang, Y.; Wang, J.; Yang, J.; Ishida, T.; Jiang, W.; Han, X.; Kang, J.; Wang, X.; et al. CLE9 Peptide-Induced Stomatal Closure is Mediated by Abscisic Acid, Hydrogen Peroxide, and Nitric Oxide in Arabidopsis thaliana. Plant Cell Environ. 2019, 42, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric Oxide and Hydrogen Sulfide Coordinately Reduce Glucose Sensitivity and Decrease Oxidative Stress via Ascorbate-Glutathione Cycle in Heat-Stressed Wheat (Triticum aestivum L.) Plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Elbasan, F.; Kucukoduk, M.; Turkan, I. Hydrogen Sulfide (H2S) and Nitric Oxide (NO) Alleviate Cobalt Toxicity in Wheat (Triticum aestivum L.) by Modulating Photosynthesis, Chloroplastic Redox and Antioxidant Capacity. J. Hazard. Mater. 2020, 388, 122061. [Google Scholar] [CrossRef] [PubMed]
- Reiter, T.A. NO• Chemistry: A Diversity of Targets in the Cell. Redox Rep. 2006, 11, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric Oxide-Induced Synthesis of Hydrogen Sulfide Alleviates Osmotic Stress in Wheat Seedlings Through Sustaining Antioxidant Enzymes, Osmolyte Accumulation and Cysteine Homeostasis. Nitric Oxide 2017, 68, 91–102. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Bahador, M.; Razmjoo, J. Investigation of the Proline Role in Controlling Traits Related to Sugar and Root Yield of Sugar Beet Under Water Deficit Conditions. Agric. Water Manag. 2021, 243, 106448. [Google Scholar] [CrossRef]
- Santisree, P.; Bhatnagar-Mathur, P.; Sharma, K.K. NO to Drought-Multifunctional Role of Nitric Oxide in Plant Drought: Do We Have All the Answers? Plant Sci. 2015, 239, 44–55. [Google Scholar] [CrossRef]
- Santisree, P.; Sanivarapu, H.; Gundavarapu, S.; Sharma, K.K.; Bhatnagar-Mathur, P. Nitric Oxide as a Signal in Inducing Secondary Metabolites During Plant Stress. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Merillon, J.M., Ramawat, K.G., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 593–621. [Google Scholar] [CrossRef]
- Cai, W.; Liu, W.; Wang, W.S.; Fu, Z.W.; Han, T.T.; Lu, Y.T. Overexpression of Rat Neurons Nitric Oxide Synthase in Rice Enhances Drought and Salt Tolerance. PLoS ONE 2015, 10, e0131599. [Google Scholar] [CrossRef]
- Bhaskarla, V.; Zinta, G.; Ford, R.; Jain, M.; Varshney, R.K.; Mantri, N. Comparative Root Transcriptomics Provide Insights into Drought Adaptation Strategies in Chickpea (Cicer arietinum L.). Int. J. Mol. Sci. 2020, 21, 1781. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.V. Mitotic Cycle and its Regulation. Vavilov J. Genet. Breed. 2014, 18, 81–92. (In Russian) [Google Scholar]
- Viola, I.L.; Güttlein, L.N.; Gonzalez, D.H. Redox Modulation of Plant Developmental Regulators from the Class I TCP Transcription Factor Family. Plant Physiol. 2013, 162, 1434–1447. [Google Scholar] [CrossRef]
- Viola, I.L.; Alem, A.L.; Jure, R.M.; Gonzalez, D.H. Physiological Roles and Mechanisms of Action of Class I TCP Transcription Factors. Int. J. Mol. Sci. 2023, 24, 5437. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root Anatomy and Soil Resource Capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Beyer, Y.; Fridovich, I. Assaying for Superoxide Dismutase Activity: Some Large Consequences of Minor Changes in Conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Verma, S.; Dubey, R.S. Lead Toxicity Induces Lipid Peroxidation and Alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Minibayeva, F.V.; Gordon, L.K.; Kolesnikov, O.P.; Chasov, A.V. Role of Extracellular Peroxidase in the Superoxide Production by Wheat Root Cells. Protoplasma 2001, 217, 125–128. [Google Scholar] [CrossRef]
- Chaitanya, K.K.; Naithani, S.C. Role of Superoxide, Lipid Peroxidation and Superoxide Dismutase in Membrane Perturbation During Loss of Viability in Seeds of Shorea robusta Gaertn. f. New Phytol. 1994, 126, 623–627. [Google Scholar] [CrossRef]
- Khairullin, R.M.; Yarullina, L.G.; Troshina, N.B.; Akhmetova, I.E. Chitooligosacharide-Induced Activation of o-Phenylenediamine Oxidation by Wheat Seedlings in the Presence of Oxalic Acid. Biochemistry 2001, 66, 286–289. [Google Scholar] [PubMed]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 Induced by Oligogalacturonides is not Involved in the Inhibition of the Auxin-Regulated rolB Gene Expression in Tobacco Leaf Explants. Plant Physiol. 2000, 122, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldran, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water Stress Studies. Plant Soil 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Citrus Plants Exude Proline and Phytohormones Under Abiotic Stress Conditions. Plant Cell Rep. 2017, 36, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kubiś, J. Involvement of Nitric Oxide in Water Stress-Induced Responses of Cucumber Roots. Plant Sci. 2009, 177, 682–690. [Google Scholar] [CrossRef]
- Pompella, A.; Maellaro, E.; Casini, A.F.; Comporti, M. Histochemical Detection of Lipid Peroxidation in the Liver of Bromobenzene-Poisoned Mice. Am. J. Pathol. 1987, 129, 295–301. [Google Scholar]
- Gill, M.B.; Zeng, F.; Shabala, L.; Zhang, G.; Yu, M.; Demidchik, V.; Shabala, S.; Zhou, M. Identification of QTL Related to ROS Formation Under Hypoxia and Their Association With Waterlogging and Salt tolerance in Barley. Int. J. Mol. Sci. 2019, 20, 699. [Google Scholar] [CrossRef]
- Bezrukova, M.; Kildibekova, A.; Shakirova, F. WGA Reduces the Level of Oxidative Stress in Wheat Seedlings Under Salinity. Plant Growth Regul. 2008, 54, 195–201. [Google Scholar] [CrossRef]
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When Bad Guys Become Good Ones: The Key Role of Reactive Oxygen Species and Nitric Oxide in the Plant Responses to Abiotic Stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Faizan, M.; Faraz, A.; Siddiqui, H.; Yusuf, M.; Hayat, S. Nitric Oxide-Mediated Integrative Alterations in Plant Metabolism to Confer Abiotic Stress Tolerance, NO Crosstalk with Phytohormones and NO-Mediated Post Translational Modifications in Modulating Diverse Plant Stress. Nitric Oxide 2018, 73, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Allagulova, C.R.; Avalbaev, A.M.; Lubyanova, A.R.; Lastochkina, O.V.; Shakirova, F.M. Current Concepts of the Mechanisms of Nitric Oxide Formation in Plants. Russ. J. Plant Physiol. 2022, 69, 339–351. [Google Scholar] [CrossRef]
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric Oxide Function in Plant Abiotic Stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Tatar, Ö.; Brück, H.; Asch, F. Atmospheric and Soil Water Deficit Induced Changes in Chemical and Hydraulic Signals in Wheat (Triticum aestivum L.). J. Agron. Crop. Sci. 2023, 209, 242–250. [Google Scholar] [CrossRef]

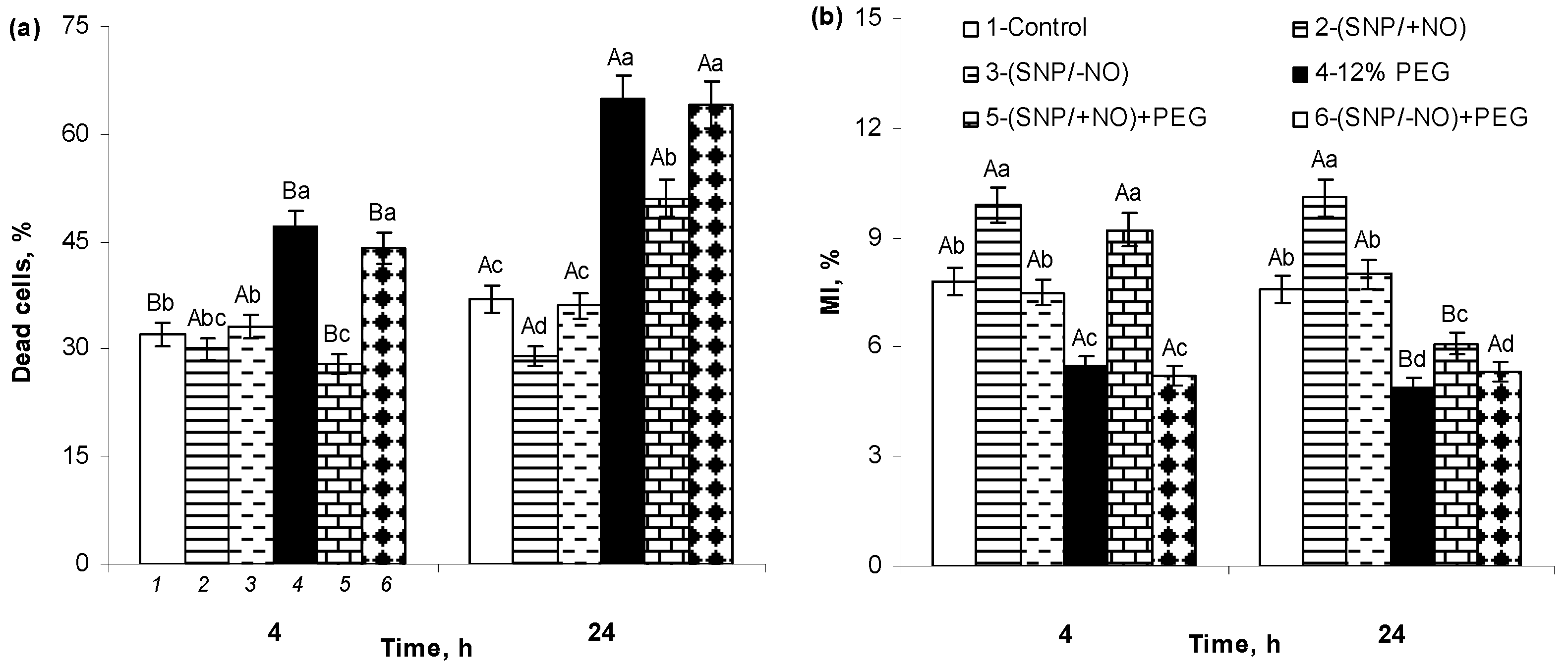
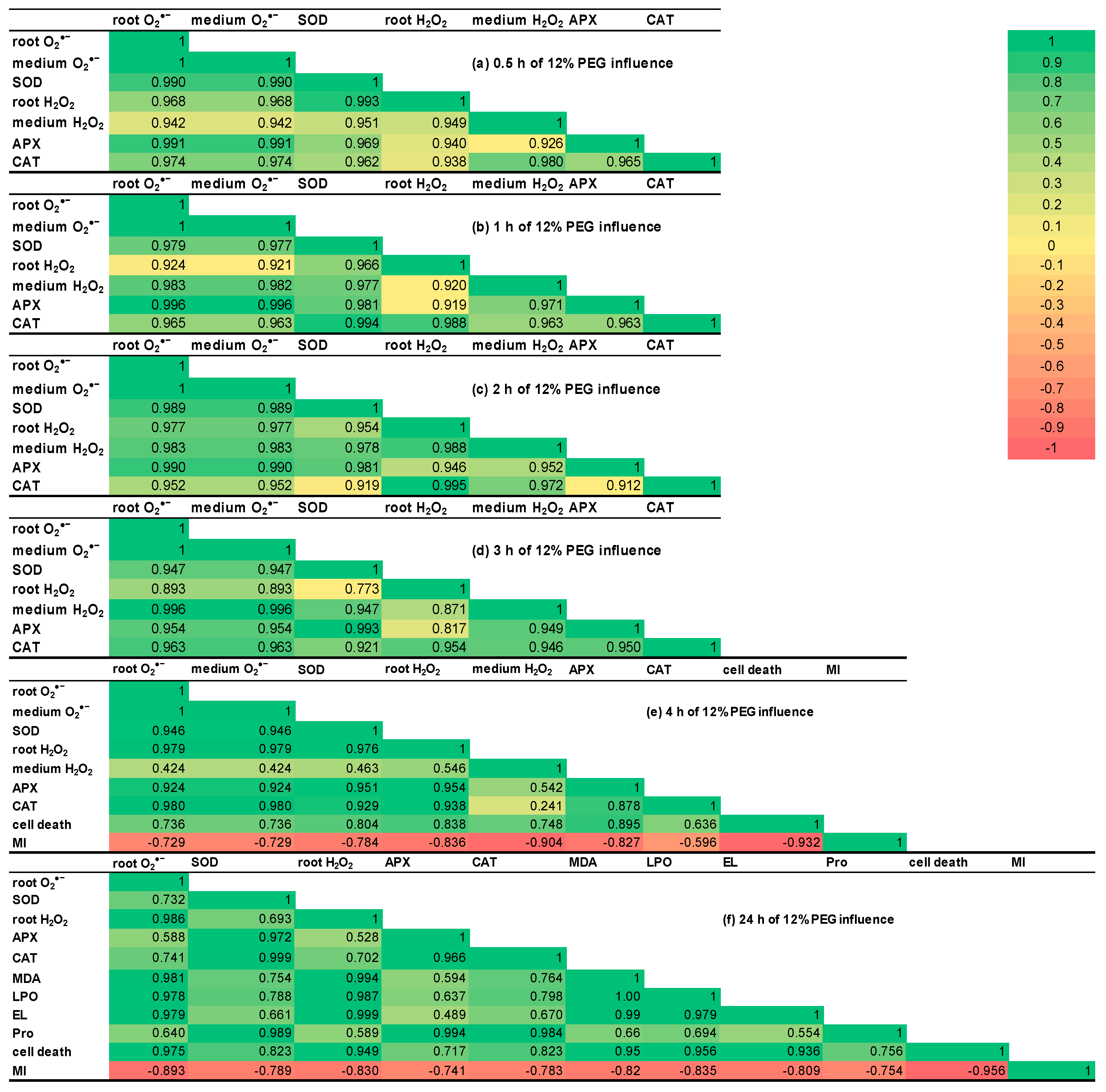

| Treatment | Root O2•− Content, OD540/(g FW × min) | Root H2O2 Content, µM/g FW | SOD Activity, Units/(mg Protein × min) | APX Activity, µM Ascorbate/(mg Protein × min) | CAT Activity, µM H2O2/(mg Protein × min) |
|---|---|---|---|---|---|
| Control | 0.34 ± 0.017 b | 153.85 ± 7.60 c | 95.9 ± 4.5 d | 1.31 ± 0.06 c | 121.8 ± 5.9 d |
| (SNP/+NO) | 0.33 ±0.019 b | 154.85 ± 7.31 c | 107.7 ± 5.2 c | 1.23 ± 0.05 c | 140.2 ± 6.7 c |
| (SNP/−NO) | 0.34 ± 0.017 b | 150.00 ± 7.40 c | 92.3 ± 4.1 d | 1.25 ± 0.06 c | 118.2 ± 5.1 d |
| 12% PEG | 0.44 ± 0.020 a | 240.00 ± 11.12 a | 236.2 ± 11.1 b | 1.71 ± 0.08 b | 273.0 ± 11.6 b |
| (SNP/+NO) + PEG | 0.37 ± 0.016 b | 175.00 ± 8.52 b | 292.2 ± 13.9 a | 2.14 ± 0.10 a | 322.8 ± 14.4 a |
| (SNP/−NO) + PEG | 0.42 ± 0.021 a | 235.06 ± 11.56 a | 240.0 ± 11.6 b | 1.74 ± 0.08 b | 268.0 ± 14.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubyanova, A.; Allagulova, C. Exogenous Sodium Nitroprusside Affects the Redox System of Wheat Roots Differentially Regulating the Activity of Antioxidant Enzymes under Short-Time Osmotic Stress. Plants 2024, 13, 1895. https://doi.org/10.3390/plants13141895
Lubyanova A, Allagulova C. Exogenous Sodium Nitroprusside Affects the Redox System of Wheat Roots Differentially Regulating the Activity of Antioxidant Enzymes under Short-Time Osmotic Stress. Plants. 2024; 13(14):1895. https://doi.org/10.3390/plants13141895
Chicago/Turabian StyleLubyanova, Alsu, and Chulpan Allagulova. 2024. "Exogenous Sodium Nitroprusside Affects the Redox System of Wheat Roots Differentially Regulating the Activity of Antioxidant Enzymes under Short-Time Osmotic Stress" Plants 13, no. 14: 1895. https://doi.org/10.3390/plants13141895






