An Integrated Approach for Biofortification of Carotenoids in Cowpea for Human Nutrition and Health
Abstract
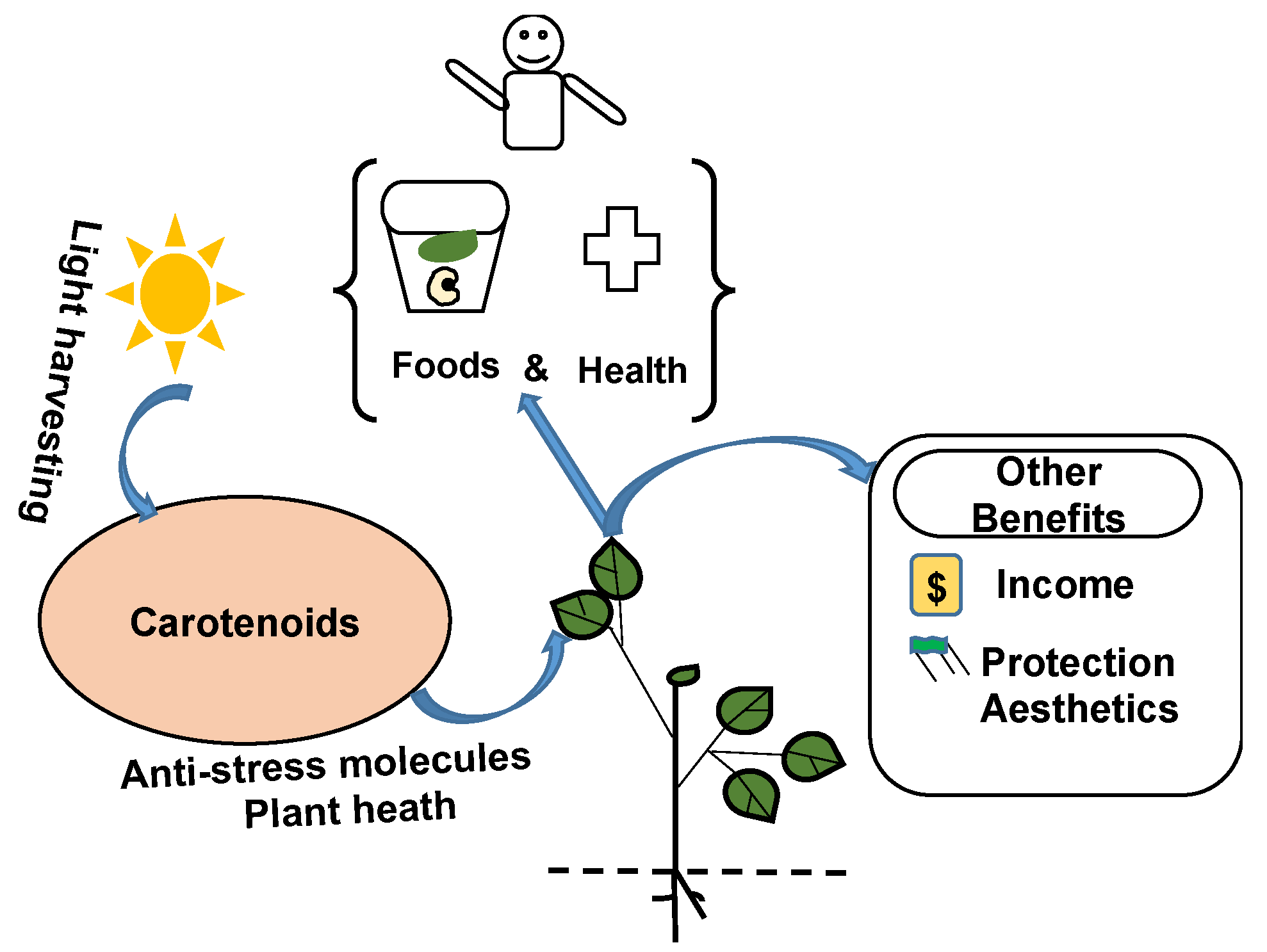
:1. Introduction
2. Importance of Carotenoids for Human Nutrition and Health
3. Carotenoid Biosynthesis in Cowpea
4. Identification and Quantification Methods of Carotenoids in Cowpea
4.1. Destructive Methods for Quantification of Carotenoids in Cowpea
4.2. Non-Destructive Analysis of Carotenoid Content in Cowpea
5. Determinants of Carotenoid Biosynthesis and Accumulation in Cowpea
5.1. Genetic, Ontogenic, and Morphological Basis of Carotenoid Variation in Cowpea
| Plant Organs | Quantification Methods | Total Carotenoids | α Carotene | β Carotene | Lutein | Zeaxanthin | Cryptoxanthin | Authors |
|---|---|---|---|---|---|---|---|---|
| µg/g | ||||||||
| Seeds | Spectrophotometry | - | - | 0.1 | - | - | [73] | |
| HPLC (C30 Column) | 9.46 | - | - | 4.3 | 5.5 | - | [15] | |
| HPLC (C18 Column) | 0.6 | - | - | 0.6 | 0 | - | [25] | |
| 0.95 | - | 0.04 | 0.9 | <0.01 | - | [69] | ||
| 0.6 | 0 | 0.06 | 0.5 | - | 0.03 | [58] | ||
| Leaves | Spectrophotometry | 436.8 | - | - | - | - | - | [70] |
| HPLC (C18 Column) | - | - | 806.0 | - | - | - | [26] | |
| HPLC (C30 Column) | 570 | 7.2 | 184.5 | 360 | 18.6 | 3.3 | [54] | |
| UHPLC (C30 Column) | 2245 | - | 958 | 1246 | 10 | - | [40] | |
| Sprouts | Spectrophotometry | - | - | 0.2 | - | - | - | [73] |
| HPLC (C18 Column) | 16.7 | 2.1 | 2.8 | 2.5 | - | 0.17 | [58] | |
| HPLC (C30 Column) | 253.7 | 5.9 | 66 | 162.1 | - | - | [41] | |
| - | - | 652 | 1824 | 393 | - | [55] | ||
5.2. Exogenous Factors Influencing Carotenoid Biosynthesis in Cowpea
- Light exposure and intensity
- Temperature
- Plant nutrition and carotenoid biosynthesis
- Plant hormones and carotenoids
| Elicitors | Crops | Growth Stage | Treatments | Treatment Duration | Effects | Authors |
|---|---|---|---|---|---|---|
| NaCl | Cowpea | 1-week-old seedlings | 60–200 mM | 14 Days | Reduce total carotenoids | [93] |
| Broad Bean | 6-week-old seedlings | 60–240 mM | 10 Days | Reduce total carotenoids | [94] | |
| Common bean | 3-week-old seedlings | 50–200 mM | 7 Days | Reduce total carotenoids | [104] | |
| Mungbean | 1-week-old seedlings | 200–250 mM | 14 Days | Reduce total carotenoids | [95] | |
| UVB | Cowpea | Germinated seeds | 470 nm | 14 Days | Increase profiles of all carotenoids | [41] |
| Fluorescence | Mungbean | Germinated seeds | 400–700 nm | 5 Days | Increase total carotenoid content | [105] |
| Dark | Mungbean Soybean | Germinated seeds | Dark conditions | 5 Days | No positive effect on carotenoids compared to light treatment | [105,106] |
6. Integrated Approach for Carotenoid Biofortification in Cowpea
6.1. Breeding for Increased Carotenoid Content in Cowpea
6.2. Harnessing the Power of Plant Factory System, Speed Breeding, and Omics
6.3. Genetic Engineering for Increasing Carotenoid Content in Cowpea
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotz, C.; McClafferty, B. From harvest to health: Challenges for developing biofortified staple foods and determining their impact on micronutrient status. Food Nutr. Bull. 2007, 28, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Varshney, R.K.; Mohan, S.M.; Gaur, P.M.; Gangarao, N.V.P.R.; Pandey, M.K.; Bohra, A.; Sawargaonkar, S.L.; Chitikineni, A.; Kimurto, P.K.; Janila, P.; et al. Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics. Biotechnol. Adv. 2013, 31, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.W. Nature and distribution of carotenoids. Food Chem. 1980, 5, 3–13. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Armstrong, G.A.; Hearstt, J.E. Gernetics and molecular biology of carotenoid pigment biosynthesis. Plant Cell 1996, 10, 228–237. [Google Scholar]
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-Perles, R.; Trindade, H.; Rosa, E.A.S.; Ferreira, L.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agri-food system: Nutritional advantages and constraints. J. Sci. Food Agric. 2016, 96, 2941–2951. [Google Scholar] [CrossRef]
- Andersson, M. Progress update: Crop development of biofortified staple food crops under HarvestPlus. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 11905–11935. [Google Scholar] [CrossRef]
- Roorkiwal, M.; Pandey, S.; Thavarajah, D.; Hemalatha, R.; Varshney, R.K. Molecular Mechanisms and Biochemical Pathways for Micronutrient Acquisition and Storage in Legumes to Support Biofortification for Nutritional Security. Front. Plant Sci. 2021, 12, 682842. [Google Scholar] [CrossRef]
- Rehman, H.M.; Cooper, J.W.; Lam, H.M.; Yang, S.H. Legume biofortification is an underexploited strategy for combatting hidden hunger. Plant Cell Environ. 2019, 42, 52–70. [Google Scholar] [CrossRef]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef]
- WHO. Vitamin A Deficiency; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Carvalho, M.; Lino-Neto, T.; Rosa, E.; Carnide, V. Cowpea: A legume crop for a challenging environment. J. Sci. Food Agric. 2017, 97, 4273–4284. [Google Scholar] [CrossRef]
- Sommer, A.; Vyas, K.S. A global clinical view on vitamin A and carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Dhaliwal, S.S. Zinc biofortification of dual-purpose cowpea [Vigna unguiculata (L.) Walp.] for enhancing the productivity and nutritional quality in a semi-arid regions of India. Arch. Agron. Soil Sci. 2021, 68, 1034–1048. [Google Scholar] [CrossRef]
- HarvestPlus. Biofortification Progress Briefs. 2014. Available online: https://www.harvestplus.org/wp-content/uploads/2014/09/Biofortification_Progress_Briefs_August2014_WEB_2_0.pdf (accessed on 21 March 2021).
- Cuttriss, A.J.; Cazzonelli, C.I.; Wurtzel, E.T.; Pogson, B.J. Carotenoids; Academic Press: Cambridge, MA, USA, 2011; Volume 58, pp. 1–36. [Google Scholar]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N.; Raghavan, V. Plant carotenoids evolution during cultivation, postharvest storage, and food processing: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1561–1604. [Google Scholar] [CrossRef]
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; King, C.A.; Fritschi, F.B. Association mapping of total carotenoids in diverse soybean genotypes based on leaf extracts and high-throughput canopy spectral reflectance measurements. PLoS ONE 2015, 10, e0137213. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Ross, A.C. Vitamin A. In Bioactive Compounds and Cancer; Humana Press: New York, NY, USA, 2010; pp. 335–356. [Google Scholar]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marín, B.; Milla, R.; Martín-Robles, N.; Arc, E.; Kranner, I.; Becerril, J.M.; García-Plazaola, J.I. Side-effects of domestication: Cultivated legume seeds contain similar tocopherols and fatty acids but less carotenoids than their wild counterparts. BMC Plant Biol. 2014, 14, 1599. [Google Scholar] [CrossRef] [PubMed]
- Nawiri, M.P.; Nyambaka, H.; Murungi, J.I. Sun-dried cowpeas and amaranth leaves recipe improves beta-carotene and retinol levels in serum and hemoglobin concentration among preschool children. Eur. J. Nutr. 2013, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- NIH. Vitamin A. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 21 March 2021).
- Clagett-Dame, M.; Knutson, D. Vitamin A in reproduction and development. Nutrients 2011, 3, 385–428. [Google Scholar] [CrossRef] [PubMed]
- Azaïs-Braesco, V.; Pascal, G. Vitamin A in pregnancy: Requirements and safety limits. Am. J. Clin. Nutr. 2000, 71, 1325S–1333S. [Google Scholar] [CrossRef] [PubMed]
- MPharm, T.P.A.; DPharm, P.A.d.R.F. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceutical. J. Cosmet. Dematol. 2012, 11, 51–54. [Google Scholar]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A.; Yildiz, F. Chemistry, encapsulation, and health benefits of β-carotene—A review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef]
- Hannoufa, A.; Hossain, Z. Carotenoid Biosynthesis and Regulation in Plants. Carotenoids Nutr. Anal. Technol. 2016, 2016, 159–189. [Google Scholar] [CrossRef]
- Rodrıguez-Concepciόn, M.; Boronat, A. Elucidation of the Methylerythritol Phosphate Pathway for Isoprenoid Biosynthesis in Bacteria and Plastids. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Saeed ur, R.; Bilal, M.; Iqbal, H.M.N.; Huang, D. Biosynthesis and biomedical perspectives of carotenoids with special reference to human health-related applications. Biocatal. Agric. Biotechnol. 2019, 17, 399–407. [Google Scholar] [CrossRef]
- Seroczyńska, A.; Korzeniewska, A.; Sztangret-wiśniewska, J.; Niemirowicz-szczytt, K.; Gajewski, M. Relationship between carotenoids content and flower or fruit flesh colour of winter squash (Cucurbita maxima Duch.). Folia Hortic. 2006, 18, 51–61. [Google Scholar]
- Arathi, B.P.; Sowmya, P.R.R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Metabolomics of carotenoids: The challenges and prospects—A review. Trends Food Sci. Technol. 2015, 45, 105–117. [Google Scholar] [CrossRef]
- Moloto, M.R.; Phan, A.D.T.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. Comparison of Phenolic Compounds, Carotenoids, Amino Acid Composition, In Vitro Antioxidant and Anti-Diabetic Activities in the Leaves of Seven Cowpea (Vigna unguiculata) Cultivars. Foods 2020, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.J.; Park, C.H.; Lee, K.B.; Kim, J.K.; Park, J.S.; Lee, J.-W.; Park, S.U. Metabolic Analysis of Vigna unguiculata Sprouts Exposed to Different Light-Emitting Diodes. Nat. Prod. Commun. 2018, 13, 1934578X1801301029. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, S.; Anees, A. Green Extraction methods and Environmental applications of Carotenoids—A Review. R. Soc. Chem. 2015, 5, 62358–62393. [Google Scholar] [CrossRef]
- Mduma, I.; Msuya, J.; Mwanri, A.W.; Yang, R.Y. Carotenoids retention and in vitro iron bioavailability of traditional cowpea leaf dishes of rural Tanzania. Int. J. Food Sci. Nutr. 2012, 63, 267–272. [Google Scholar] [CrossRef]
- Arvayo-Enríquez, H.; Mondaca-Fernández, I.; Gortárez-Moroyoqui, P.; López-Cervantes, J.; Rodríguez-Ramírez, R. Carotenoids extraction and quantification: A review. Anal. Methods 2013, 5, 2916–2924. [Google Scholar] [CrossRef]
- Liu, G.N.; Zhu, Y.H.; Jiang, J.G. The metabolomics of carotenoids in engineered cell factory. Appl. Microbiol. Biotechnol. 2009, 83, 989–999. [Google Scholar] [CrossRef]
- Kopec, R.E.; Cooperstone, J.L.C.; Morgan, J.; Schwartz, H.F. Methods of analysis (extraction, separation, identification and quantification) of carotenoids from natural products. In Analysis of Antioxidant-Rich Phytochemicals, 1st ed.; Xu, Z., Howard, L.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rodriguez-amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; p. 64. [Google Scholar]
- Duke, S.O.; Kenyon, W.H. Effects of dimethazone (FMC 57020) on chloroplast development: II. Pigment synthesis and photosynthetic function in cowpea (Vigna unguiculata L.) primary leaves. Pestic. Biochem. Physiol. 1986, 25, 11–18. [Google Scholar] [CrossRef]
- Nyambaka, H.; Ryley, J. An isocratic reversed-phase HPLC separation of the stereoisomers of the provitamin A carotenoids (α-and β-carotene) in dark green vegetables. Food Chem. 1996, 55, 63–72. [Google Scholar] [CrossRef]
- Mkambula, P.; Mbuya, M.N.N.; Rowe, L.A.; Sablah, M.; Friesen, V.M.; Chadha, M.; Osei, A.K.; Ringholz, C.; Vasta, F.C.; Gorstein, J. The Unfinished Agenda for Food Fortification in Low- and Middle-Income Countries: Quantifying Progress, Gaps and Potential Opportunities. Nutrients 2020, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Murkovic, M. Thin-layer chromatographic analysis of carotenoids in plant and animal samples. J. Planar Chromatogr. Mod. TLC 2010, 23, 94–103. [Google Scholar] [CrossRef]
- Weber, E.J. Carotenoids and tocols of corn grain determined by HPLC. J. Am. Oil Chem. Soc. 1987, 64, 1129–1134. [Google Scholar] [CrossRef]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding crops for better nutrition. Crop Sci. 2007, 47, S-88–S-105. [Google Scholar] [CrossRef]
- Djuikwo, V.N.D.; Ejoh, R.A.; Gouado, I.; Mbofung, C.M.; Tanumihardjo, S.A. Determination of Major Carotenoids in Processed Tropical Leafy Vegetables Indigenous to Africa. Food Nutr. Sci. 2011, 2, 793–802. [Google Scholar] [CrossRef]
- Sodedji, F.A.K.; Ryu, D.; Choi, J.; Agbahoungba, S.; Assogbadjo, A.E.; N’Guetta, S.-P.A.; Jung, J.H.; Nho, C.W.; Hoyoun, K. Genetic Diversity and Association Analysis for Carotenoid Content among Sprouts of Cowpea (Vigna unguiculata L. Walp.). Int. J. Mol. Sci. 2022, 23, 3696. [Google Scholar] [CrossRef]
- Bijttebier, S.; D’Hondt, E.; Noten, B.; Hermans, N.; Apers, S.; Voorspoels, S. Ultra high performance liquid chromatography versus high performance liquid chromatography: Stationary phase selectivity for generic carotenoid screening. J. Chromatogr. A 2014, 1332, 46–56. [Google Scholar] [CrossRef]
- Seke, F.; Moloto, M.R.; Shoko, T.; Sultanbawa, Y.; Sivakumar, D. Comparative study of the functional compounds and antioxidant properties of different cowpea (Vigna unguiculata) leaf cultivars after in vitro digestion. Int. J. Food Sci. Technol. 2022, 58, 1089–1097. [Google Scholar] [CrossRef]
- Jirapa, P.; Normah, H.; Zamaliah, M.M.; Asmah, R.; Mohamad, K. Nutritional quality of germinated cowpea flour (Vigna unguiculata) and its application in home prepared powdered weaning foods. Plant Foods Hum. Nutr. 2001, 3, 203–216. [Google Scholar] [CrossRef]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef]
- Awad, H.; Khamis, M.M.; El-Aneed, A. Mass Spectrometry, Review of the Basics: Ionization. Appl. Spectrosc. Rev. 2014, 50, 158–175. [Google Scholar] [CrossRef]
- Neugart, S.; Baldermann, S.; Ngwene, B.; Wesonga, J.; Schreiner, M. Indigenous leafy vegetables of Eastern Africa—A source of extraordinary secondary plant metabolites. Food Res. Int. 2017, 100, 411–422. [Google Scholar] [CrossRef]
- Pedro, A.M.K.; Ferreira, M.R.M.C. Nondestructive Determination of Solids and Carotenoids in Tomato Products by Near-Infrared Spectroscopy and Multivariate Calibration. Anal. Chem. 2005, 77, 2505–2511. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, X.; Kong, W.; Ye, H. Monitoring Crop Carotenoids Concentration by Remote Sensing; IntechOpen: London, UK, 2018; pp. 1198–1217. [Google Scholar]
- Hempel, J.; Müller-Maatsch, J.; Carle, R.; Schweiggert, R.M. Non-destructive approach for the characterization of the in situ carotenoid deposition in gac fruit aril. J. Food Compos. Anal. 2018, 65, 16–22. [Google Scholar] [CrossRef]
- Brenna, O.V.; Berardo, N. Application of Near-Infrared Reflectance Spectroscopy (NIRS) tothe Evaluation of Carotenoids Content in Maize. J. Agric. Food Chem. 2004, 52, 5577–5582. [Google Scholar] [CrossRef] [PubMed]
- Abincha, W.; Ikeogu, U.N.; Kawuki, R.; Egesi, C.; Rabbi, I.; Parkes, E.; Kulakow, P.; Edema, R.; Gibson, P.; Owor, B.-E. Portable Spectroscopy Calibration with Inexpensive and Simple Sampling Reference Alternatives for Dry Matter and Total Carotenoid Contents in Cassava Roots. Appl. Sci. 2021, 11, 1714. [Google Scholar] [CrossRef]
- Gemenet, D.C.; da Silva Pereira, G.; De Boeck, B.; Wood, J.C.; Mollinari, M.; Olukolu, B.A.; Diaz, F.; Mosquera, V.; Ssali, R.T.; David, M.; et al. Quantitative trait loci and differential gene expression analyses reveal the genetic basis for negatively associated beta-carotene and starch content in hexaploid sweetpotato [Ipomoea batatas (L.) Lam.]. Theor. Appl. Genet. 2020, 133, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Mamatha, B.S.; Sangeetha, R.K.; Baskaran, V. Provitamin-A and xanthophyll carotenoids in vegetables and food grains of nutritional and medicinal importance. Int. J. Food Sci. Technol. 2011, 46, 315–323. [Google Scholar] [CrossRef]
- Natabirwa, H.; Mukiibi, J.; Zziwa, E.; Kabirizi, J. Nutritional and physicochemical properties of stored solar-dried cowpea leafy vegetables. Uganda J. Agric. Sci. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Cunningham, F.X. Regulation of carotenoid synthesis and accumulation in plants. Pure Appl. Chem. 2002, 74, 1409–1417. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Míguez, F.; Méndez-Fernández, L.; Agut, A.; Becerril, J.M.; García-Plazaola, J.I.; Kranner, I.; Colville, L. Seed carotenoid and tocochromanol composition of wild fabaceae species is shaped by phylogeny and ecological factors. Front. Plant Sci. 2017, 8, 1428. [Google Scholar] [CrossRef] [PubMed]
- Luthria, A.; Singh, K.; D’Souza, M. In Vitro Antioxidant Activity of Black Gram, Cowpea, Desi Chickpea and Yellow Mustard as Affected by Sprouting. J. Glob. Biosci. 2014, 3, 1355–2320. [Google Scholar]
- Karapanos, I.; Papandreou, A.; Skouloudi, M.; Makrogianni, D.; Fernández, J.A.; Rosa, E.; Ntatsi, G.; Bebeli, P.J.; Savvas, D. Cowpea fresh pods—A new legume for the market: Assessment of their quality and dietary characteristics of 37 cowpea accessions grown in southern Europe. J. Sci. Food Agric. 2017, 97, 4343–4352. [Google Scholar] [CrossRef] [PubMed]
- Kantha, S.S.; Erdman, J.W. Legume carotenoids. CRC Crit. Rev. Food Sci. Nutr. 1987, 26, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Monma, M.; Terao, J.; Lto, M.; Saito, M.; Chikuni, K. Carotenoid Components in Soybean Seeds Varying with Seed Color and Maturation Stage. Biosci. Biotechnol. Biochem. 1994, 58, 926–930. [Google Scholar] [CrossRef]
- Herniter, I.A.; Lo, R.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.-N.; Huynh, B.-L.; Lucas, M.; Jia, Z.; Roberts, P.A.; Lonardi, S.; et al. Seed Coat Pattern QTL and Development in Cowpea [Vigna unguiculata (L.) Walp.]. Front. Plant Sci. 2019, 10, 1346. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Banerjee, N.; Noratto, G.D.; Angel-Morales, G.; Hachibamba, T.; Awika, J.M.; Mertens-Talcott, S.U. Polyphenolic extracts from cowpea (Vigna unguiculata) protect colonic myofibroblasts (CCD18Co cells) from lipopolysaccharide (LPS)-induced inflammation—Modulation of microRNA 126. Food Funct. 2015, 6, 146–154. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Yang, L.; Dykes, L.; Awika, J. Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O-glucoside as the dominant compound. Food Chem. 2013, 139, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Srivastava, G.; Prasad, S.M.; Abraham, G. Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate. Pestic. Biochem. Physiol. 2008, 92, 30–37. [Google Scholar] [CrossRef]
- dos Santos, E.R.; Borges, P.R.S.; Siebeneichler, S.C.; de Cerqueira, A.P.; Pereira, P.R. Growth and yield of leaf pigments in cowpea beans grown in two light environments. Rev. Caatinga 2011, 24, 14–19. [Google Scholar]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Lefsrud, M.G.; Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Air Temperature Affects Biomass and Carotenoid Pigment Accumulation in Kale and Spinach Grown in a Controlled Environment. HortScience 2005, 40, 2026–2030. [Google Scholar] [CrossRef]
- Singh, S.K.; Kakani, V.G.; Surabhi, G.K.; Reddy, K.R. Cowpea (Vigna unguiculata [L.] Walp.) genotypes response to multiple abiotic stresses. J. Photochem. Photobiol. B 2010, 100, 135–146. [Google Scholar] [CrossRef]
- McDonald, G.K.; Paulsen, G.M. High temperature effects on photosynthesis and water relations of grain legumes. Plant Soil 1997, 196, 47–58. [Google Scholar] [CrossRef]
- Ismail, A.M.; Hall, A.E. Positive and potential negative effects of heat-tolerance genes in cowpea. Crop Sci. 1998, 38, 381–390. [Google Scholar] [CrossRef]
- Jones, A. Heat Stress in Legume Seed Setting: Effects, Causes, and Future Prospects. Front. Plant Sci. 2019, 10, 938. [Google Scholar] [CrossRef]
- Kaur, R.; Bains, T.S.; Bindumadhava, H.; Nayyar, H. Responses of mungbean (Vigna radiata L.) genotypes to heat stress: Effects on reproductive biology, leaf function and yield traits. Sci. Hortic. 2015, 197, 527–541. [Google Scholar] [CrossRef]
- Roy, R.N.; Finck, A.; Blair, G.J.; Tandon, H.L.S. Plant Nutrition for Food Security: A Guide for Integrated Nutrient Management; FAO: Rome, Italy, 2006; Volume 16, p. 348. [Google Scholar]
- Tanuja, S.; Nayak, S.K.; Sadangi, D.N. Growth, yield and biochemical responses of cowpea (Vigna unguiculata) to fish silage enriched vermicompost. Indian J. Fish. 2019, 66, 138–141. [Google Scholar] [CrossRef]
- Cheng, W.S.; Zeng, D.L.; Zhang, N.; Zeng, H.X.; Ren, J.; Shi, X.F.; Li, Y.H.; Sun, Y.H. Expression Analysis of Salt Stress on Carotenoid Pathway Genes in Watermelon. Adv. Mater. Res. 2014, 1073–1076, 1061–1066. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Luo, H.; Li, D.; Liu, C.; Song, J.; Zhang, Z.; Liu, C.; Niu, L. Effect of NaCl stress and supplemental CaCl2 on carotenoid accumulation in germinated yellow maize kernels. Food Chem. 2020, 309, 125779. [Google Scholar] [CrossRef] [PubMed]
- Cha-um, S.; Batin, C.B.; Samphumphung, T.; Kidmanee, C. Physio-morphological changes of cowpea (Vigna unguiculata Walp.) and jack bean (Canavalia ensiformis (L.) DC.) in responses to soil salinity. Aust. J. Crop Sci. 2013, 7, 2128–2135. [Google Scholar]
- Abdul Qados, A.M.S. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J. Saudi Soc. Agric. Sci. 2011, 10, 7–15. [Google Scholar] [CrossRef]
- Alharby, H.F.; Al-Zahrani, H.S.; Hakeem, K.R.; Iqbal, M. Identification of physiological and biochemical markers for salt (NaCl) stress in the seedlings of mungbean [Vigna radiata (L.) Wilczek] genotypes. Saudi J. Biol. Sci. 2019, 26, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Mukherji, S.; Dutta, J. Effect of manganese toxicity on pigment content, Hill activity and photosynthetic rate of Vigna radiata L. Wilczek seedlings. J. Environ. Biol. 2002, 23, 253–257. [Google Scholar]
- Fahad, S.; Nie, L.; Chen, Y.; Wu, C.; Xiong, D.; Saud, S.; Hongyan, L.; Cui, K.; Huang, J. Crop Plant Hormones and Environmental Stress. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2015; pp. 371–400. [Google Scholar]
- Jamwal, K.; Bhattacharya, S.; Puri, S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants 2018, 9, 26–38. [Google Scholar] [CrossRef]
- Maruri-Lopez, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and Extracellular Journey of the Phytohormone Salicylic Acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef]
- Chandra, A.; Bhatt, R.K. Biochemical and physiological response to salicylic acid in relation to the systemic acquired resistance. Photosynthetica 1998, 35, 255–258. [Google Scholar] [CrossRef]
- Águila Ruiz-Sola, M.; Arbona, V.; Gómez-Cadenas, A.; Rodríguez-Concepción, M.; Rodríguez-Villalón, A. A root specific induction of carotenoid biosynthesis contributes to ABA production upon salt stress in arabidopsis. PLoS ONE 2014, 9, e90765. [Google Scholar] [CrossRef]
- Sabu, K.K.; Nadiya, F. Small RNA manipulation in plants: Techniques and recent developments. In Plant Small RNA; Academic Press: Cambridge, MA, USA, 2020; pp. 379–413. [Google Scholar]
- Luchi, S.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol. 2000, 123, 553–562. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Dipierro, N.; Paciolla, C. Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts. Antioxidants 2020, 9, 558. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, Y.S.; Lee, J.D.; Chang, W.S.; Choung, M.G. Metabolic alterations of lutein, beta-carotene and chlorophyll a during germination of two soybean sprout varieties. Food Chem. 2013, 141, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.; Low, J.; McEwan, M.; Tanumihardjo, S. Biofortification: Evidence and Lessons Learned Linking Agriculture and Nutrition; FAO: Rome, Italy, 2013; pp. 1–23. [Google Scholar]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, N.; Evans, A. Advances in carotenoid increments in storage parts of African staple crops. J. Plant Breed. Crop Sci. 2019, 11, 68–79. [Google Scholar] [CrossRef]
- Gedil, M.; Menkir, A. An Integrated Molecular and Conventional Breeding Scheme for Enhancing Genetic Gain in Maize in Africa. Front. Plant Sci. 2019, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, V.; Ng, Q.; Lawson, M.; Ortiz, R. Cowpea [Vigna unguiculata (L.) Walp.] core collection defined by geographical, agronomical and botanical descriptors. Plant Genet. Resour. Charact. Util. 2007, 5, 113–119. [Google Scholar] [CrossRef]
- Fatokun, C.; Girma, G.; Abberton, M.; Gedil, M.; Unachukwu, N.; Oyatomi, O.; Yusuf, M.; Rabbi, I.; Boukar, O. Genetic diversity and population structure of a mini-core subset from the world cowpea (Vigna unguiculata (L.) Walp.) germplasm collection. Sci. Rep. 2018, 8, 16035. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Amatriaín, M.; Lo, S.; Herniter, I.A.; Boukar, O.; Fatokun, C.; Carvalho, M.; Castro, I.; Guo, Y.N.; Huynh, B.L.; Roberts, P.A.; et al. The UCR Minicore: A valuable resource for cowpea research and breeding. Legume Sci. 2021, 3, e95. [Google Scholar] [CrossRef]
- Qin, J.; Shi, A.; Xiong, H.; Mou, B.; Motes, D.R.; Lu, W.; Miller, J.C.; Scheuring, D.C.; Nzaramba, M.N.; Weng, Y.; et al. Population structure analysis and association mapping of seed antioxidant content in USDA cowpea (Vigna unguiculata L. Walp.) core collection using SNPs. Can. J. Plant Sci. 2016, 96, 1026–1036. [Google Scholar] [CrossRef]
- Stanley, L.; Yuan, Y.W. Transcriptional Regulation of Carotenoid Biosynthesis in Plants: So Many Regulators, So Little Consensus. Front. Plant Sci. 2019, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.S.; Hossain, F.; Muthusamy, V.; Zunjare, R.U. Marker-Assisted Breeding for Enrichment of Provitamin A in Maize. In Quality Breeding in Field Crops; Springer: Cham, Switzerland, 2019; pp. 139–157. [Google Scholar]
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M.C.; et al. The genome of cowpea (Vigna unguiculata [L.] Walp.). Plant J. 2019, 98, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, J.T.; Gowda, B.S.; Jean, M.; Close, T.J.; Ehlers, J.D.; Hall, A.E.; Gillaspie, A.G.; Roberts, P.A.; Ismail, A.M.; Bruening, G.; et al. An improved genetic linkage map for cowpea (Vigna unguiculata L.) Combining AFLP, RFLP, RAPD, biochemical markers, and biological resistance traits. Genome 2002, 45, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, N.; Wu, Z.; Guo, R.; Yu, X.; Zheng, Y.; Xia, Q.; Gui, S.; Chen, C. A High Density Genetic Map Derived from RAD Sequencing and Its Application in QTL Analysis of Yield-Related Traits in Vigna unguiculata. Front. Plant Sci. 2017, 8, 1544. [Google Scholar] [CrossRef]
- Muchero, W.; Diop, N.N.; Bhat, P.R.; Fenton, R.D.; Wanamaker, S.; Pottorff, M.; Hearne, S.; Cisse, N.; Fatokun, C.; Ehlers, J.D.; et al. A consensus genetic map of cowpea [Vigna unguiculata (L.) Walp.] and synteny based on EST-derived SNPs. Proc. Natl. Acad. Sci. USA 2009, 106, 18159–18164. [Google Scholar] [CrossRef]
- Adetumbi, J.A.; Akinyosoye, S.T.; Olowolafe, M.O.; Oloyede-Kamiyo, Q.O.; Agbeleye, O.A. Genetic linkage map of cowpea (Vigna unguiculata (L.) Walp.) using SNP markers. Afr. J. Biotechnol. 2016, 15, 830–834. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Liu, X.; Hu, L.; Wang, S.; Cheng, X. De novo transcriptomic analysis of cowpea (Vigna unguiculata L. Walp.) for genic SSR marker development. BMC Genet. 2017, 18, 65. [Google Scholar] [CrossRef]
- Mafakheri, K.; Bihamta, M.R.; Abbasi, A.R. Assessment of genetic diversity in cowpea (Vigna unguiculata L.) germplasm using morphological and molecular characterisation. Cogent Food Agric. 2017, 3, 1327092. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gopalakrishna, T. Development of unigene-derived SSR markers in cowpea (Vigna unguiculata) and their transferability to other Vigna species. Genome 2010, 53, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Diouf, M.; Diallo, S.; Abaye Badiane, F.; Diack, O.; Diouf, D. Development of new cowpea (Vigna unguiculata) mutant genotypes, analysis of their agromorphological variation, genetic diversity and population structure. Biocell 2021, 45, 345–362. [Google Scholar] [CrossRef]
- Sharp, P.; Dong, C. Tilling for plant breeding. Methods Mol. Biol. 2014, 1145, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Muthoni, J.; Shimelis, H. Mating designs commonly used in plant breeding: A review. Aust. J. Crop Sci. 2020, 14, 1855–1869. [Google Scholar] [CrossRef]
- Cazzola, F.; Bermejo, C.J.; Gatti, I.; Cointry, E. Speed breeding in pulses: An opportunity to improve the efficiency of breeding programs. Crop Pasture Sci. 2021, 72, 165–172. [Google Scholar] [CrossRef]
- Sreekala, C.; Raghava, S.P. Exploitation of heterosis for carotenoid content in African marigold (Tagetes erecta L.) and its correlation with esterase polymorphism. Theor. Appl. Genet. 2003, 106, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kandianis, C.B.; Stevens, R.; Liu, W.; Palacios, N.; Montgomery, K.; Pixley, K.; White, W.S.; Rocheford, T. Genetic architecture controlling variation in grain carotenoid composition and concentrations in two maize populations. Theor. Appl. Genet. 2013, 126, 2879–2895. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Plant Factory as a Resource-Efficient Closed Plant Production System. In Plant Factory; Academic Press: Cambridge, MA, USA, 2016; pp. 69–90. [Google Scholar]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Frede, K.; Schreiner, M.; Zrenner, R.; Graefe, J.; Baldermann, S. Carotenoid biosynthesis of pak choi (Brassica rapa ssp. chinensis) sprouts grown under different light-emitting diodes during the diurnal course. Photochem. Photobiol. Sci. 2018, 17, 1289–1300. [Google Scholar] [CrossRef]
- Ntagkas, N.; de Vos, R.C.H.; Woltering, E.J.; Nicole, C.C.S.; Labrie, C.; Marcelis, L.F.M. Modulation of the Tomato Fruit Metabolome by LED Light. Metabolites 2020, 10, 266. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jahne, F.; Hahn, V.; Wurschum, T.; Leiser, W.L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef] [PubMed]
- Akpolat, H.; Barineau, M.; Jackson, K.A.; Akpolat, M.Z.; Francis, D.M.; Chen, Y.J.; Rodriguez-Saona, L.E. High-throughput phenotyping approach for screening major carotenoids of tomato by handheld raman spectroscopy using chemometric methods. Sensors 2020, 20, 3723. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef]
- Varshney, R.K.; Bohra, A.; Yu, J.; Graner, A.; Zhang, Q.; Sorrells, M.E. Designing Future Crops: Genomics-Assisted Breeding Comes of Age. Trends Plant Sci. 2021, 26, 631–649. [Google Scholar] [CrossRef]
- Pazhamala, L.T.; Kudapa, H.; Weckwerth, W.; Millar, A.H.; Varshney, R.K. Systems biology for crop improvement. Plant Genome 2021, 14, e20098. [Google Scholar] [CrossRef]
- Zheng, X.; Kuijer, H.N.J.; Al-Babili, S. Carotenoid Biofortification of Crops in the CRISPR Era. Trends Biotechnol. 2020, 39, 857–860. [Google Scholar] [CrossRef]
- Jha, A.B.; Warkentin, T.D. Biofortification of pulse crops: Status and future perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef]
- Mudziwapasi, R.; Ndudzo, A.; Nyamusamba, R.P.; Jomane, F.N.; Mutengwa, T.T.; Maphosa, M. Unlocking the potential of CRISPR technology for improving livelihoods in Africa. Biotechnol. Genet. Eng. Rev. 2018, 34, 198–215. [Google Scholar] [CrossRef]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision genome editing in plants: State-of-the-art in CRISPR/Cas9-based genome engineering. BMC Plant Biol. 2020, 20, 234. [Google Scholar] [CrossRef]
- Romer, S.; Lubeck, J.; Kauder, F.; Steiger, S.; Adomat, C.; Sandmann, G. Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab. Eng. 2002, 4, 263–272. [Google Scholar] [CrossRef]
- Sandmann, G. Genetic manipulation of carotenoid biosynthesis: Strategies, problems and achievements. Trends Plant Sci. 2001, 6, 14–17. [Google Scholar] [CrossRef]
- Mishiba, K.I.; Nishida, K.; Inoue, N.; Fujiwara, T.; Teranishi, S.; Iwata, Y.; Takeda, S.; Koizumi, N. Genetic engineering of eggplant accumulating beta-carotene in fruit. Plant Cell Rep. 2020, 39, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Badejo, A.A. Elevated carotenoids in staple crops: The biosynthesis, challenges and measures for target delivery. J. Genet. Eng. Biotechnol. 2018, 16, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.; Tripathi, L.; Mkoko, B.; Ofosu, D.O.; Oloka, H.; Wangari, D. Biosafety Regulatory Reviews and Leeway to Operate: Case Studies from Sub-Sahara Africa. Front. Plant Sci. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodedji, K.A.F.; Assogbadjo, A.E.; Lee, B.; Kim, H.-Y. An Integrated Approach for Biofortification of Carotenoids in Cowpea for Human Nutrition and Health. Plants 2024, 13, 412. https://doi.org/10.3390/plants13030412
Sodedji KAF, Assogbadjo AE, Lee B, Kim H-Y. An Integrated Approach for Biofortification of Carotenoids in Cowpea for Human Nutrition and Health. Plants. 2024; 13(3):412. https://doi.org/10.3390/plants13030412
Chicago/Turabian StyleSodedji, Kpedetin Ariel Frejus, Achille Ephrem Assogbadjo, Bokyung Lee, and Ho-Youn Kim. 2024. "An Integrated Approach for Biofortification of Carotenoids in Cowpea for Human Nutrition and Health" Plants 13, no. 3: 412. https://doi.org/10.3390/plants13030412





