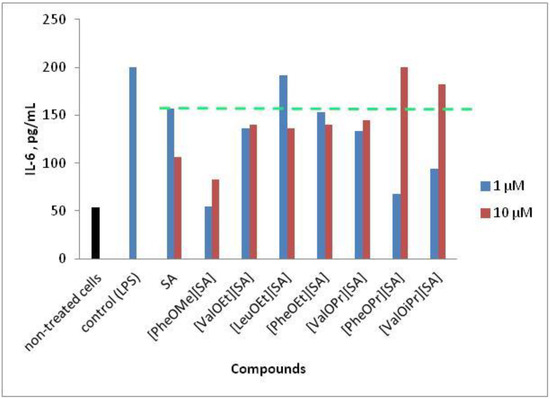
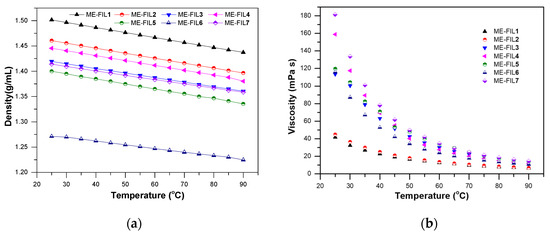
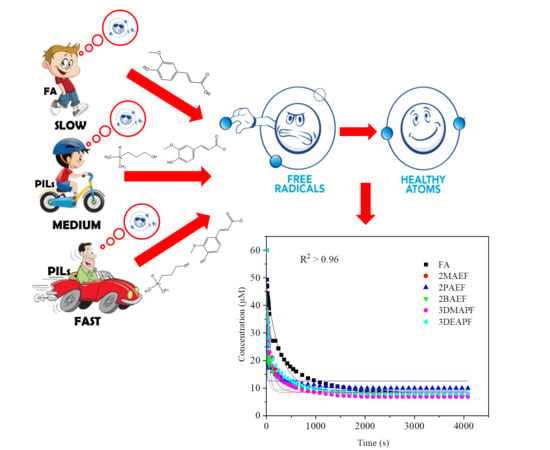
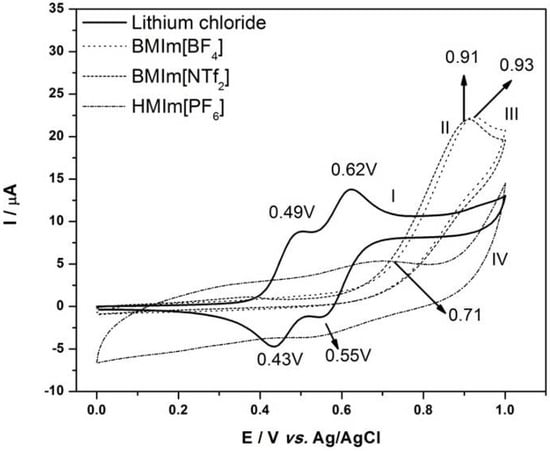
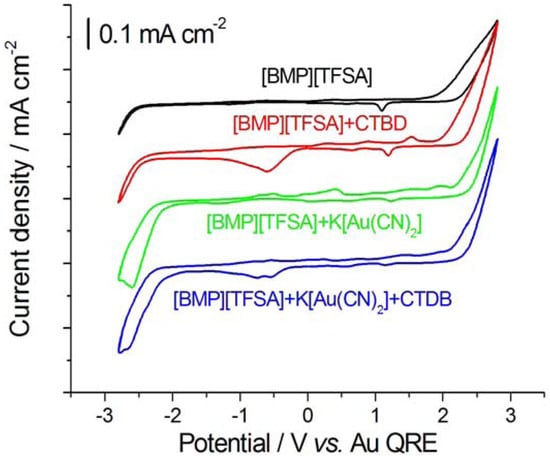
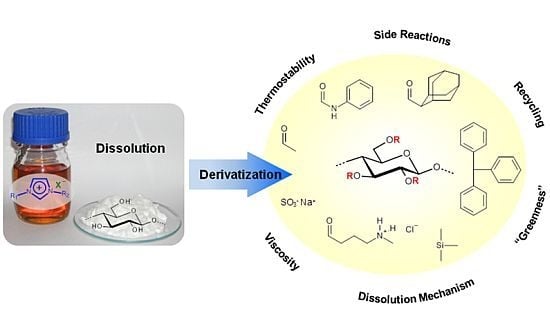
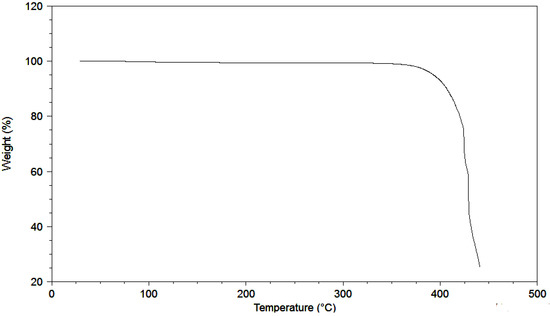
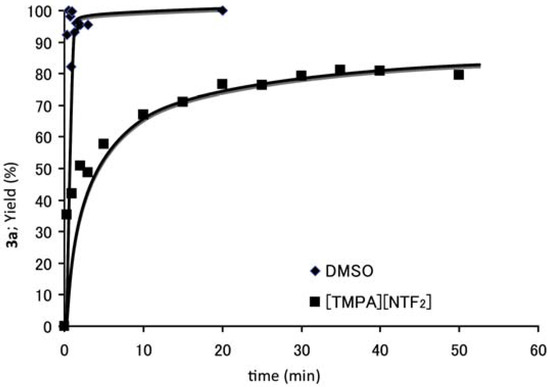
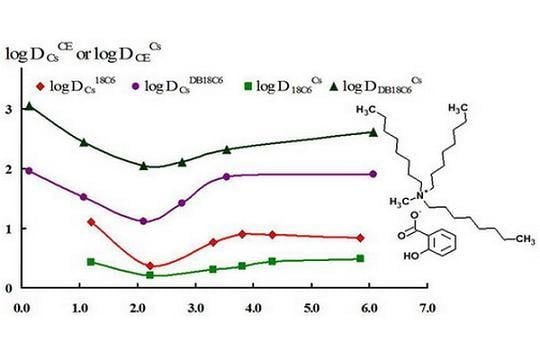
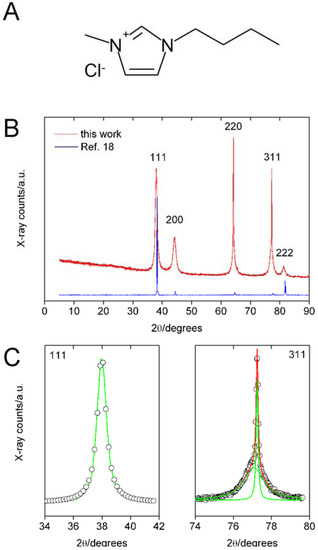
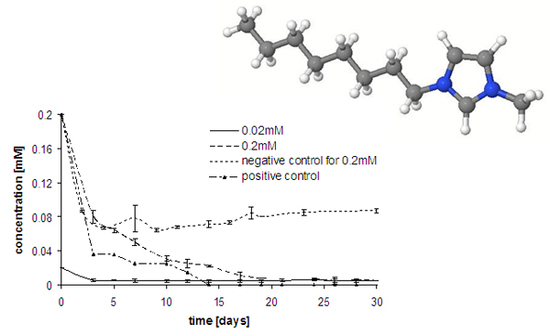
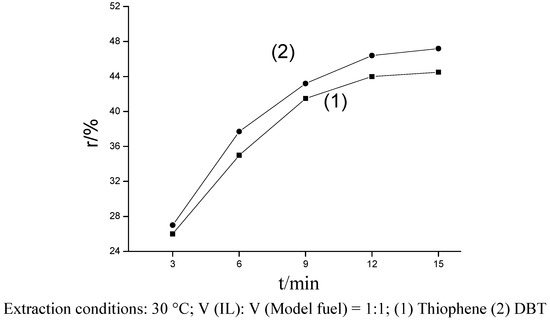
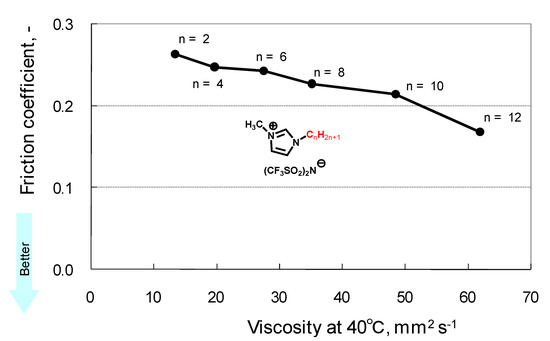
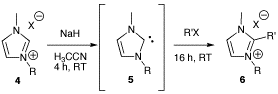
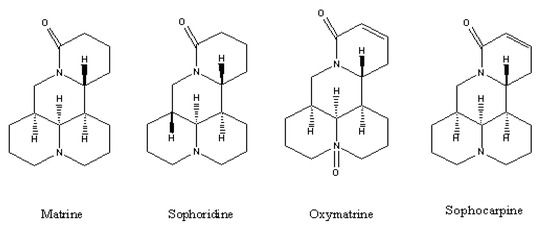
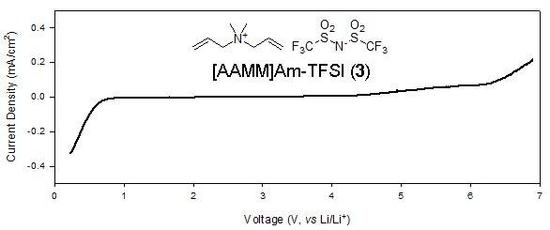
Ionic Liquids (Closed)
A topical collection in Molecules (ISSN 1420-3049).
Viewed by 348159Editor
Interests: catalysis (general); vitamins (general); chemistry under non-classical conditions (ionic liquids; supercritical fluids; ultrasound and microwaves)
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleague,
Contributions covering all the aspects indicated by the following keywords are welcomed on this collection. Both review and research papers are welcome on this collection.
- ionic liquids, molten salts
- room temperature ionic liquids
- low temperature ionic liquids
- third generation of ionic liquids
- immidazolium salts
- pyridinuim salts
- Baylis-Hillmann reaction
- Diels–Alder reactions
Dr. Werner Bonrath
Collection Editor
Related Special Issues
- Ionic Liquids in Organic Synthesis in Molecules (11 articles)
- Ionic Liquids for Chemical and Biochemical Applications in Molecules (5 articles)
- Ionic Liquids and Deep Eutectic Solvents for Synthesis, Materials and Energy in Molecules (12 articles)