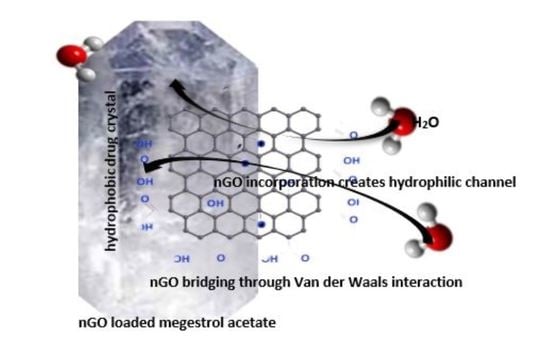
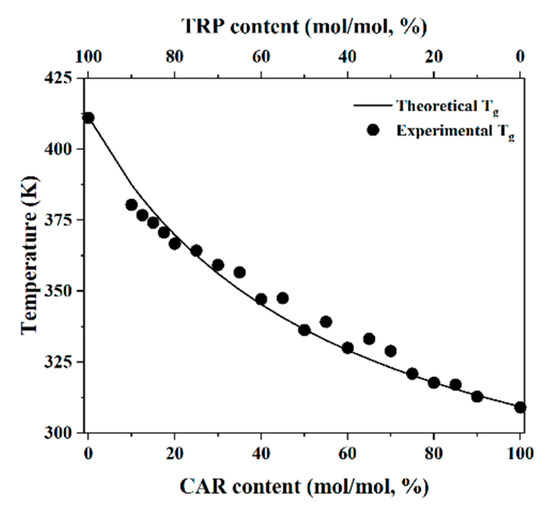
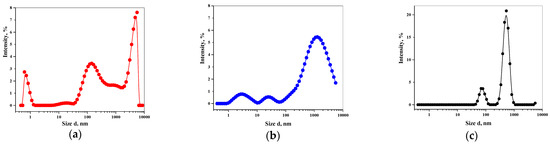
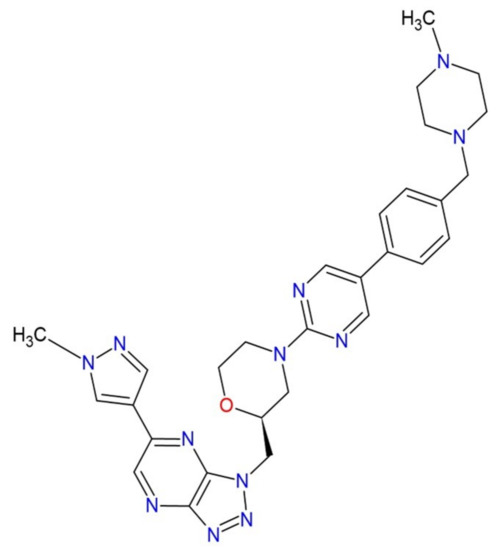
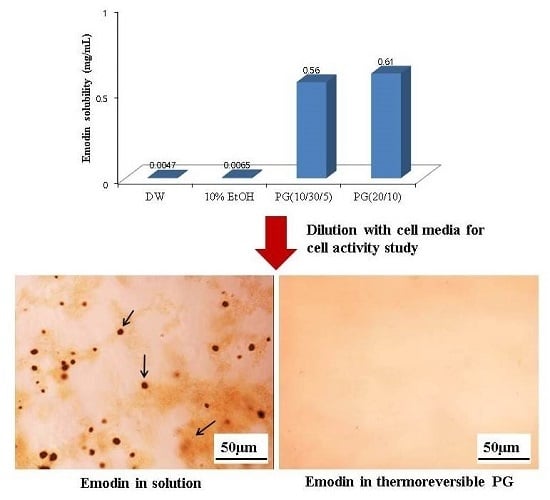
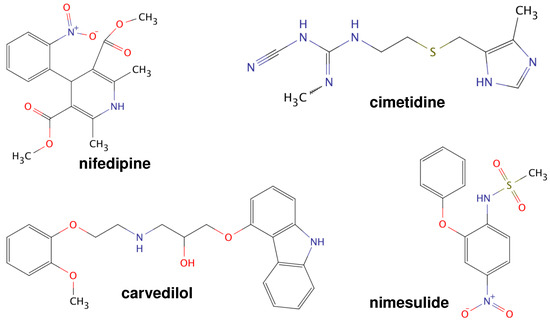
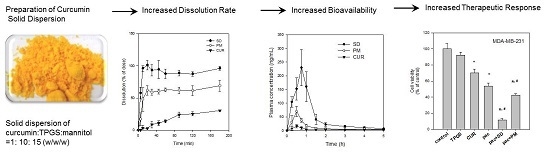
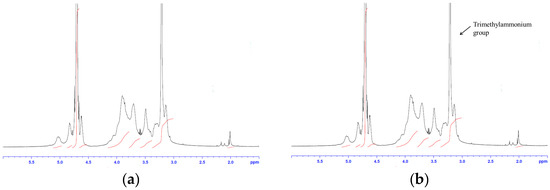
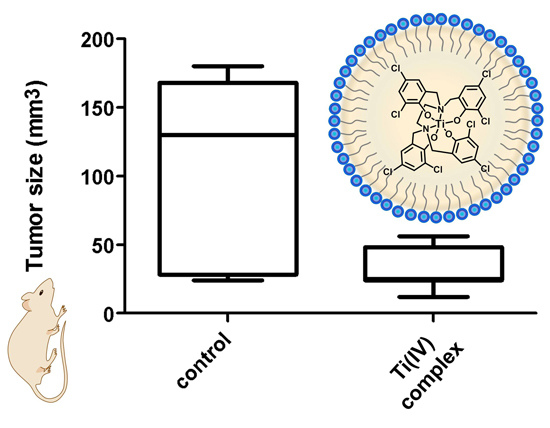
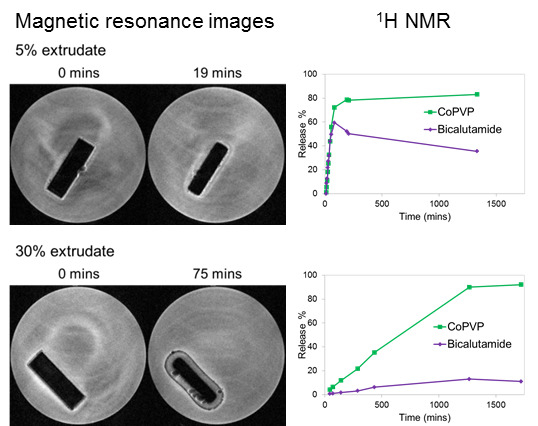
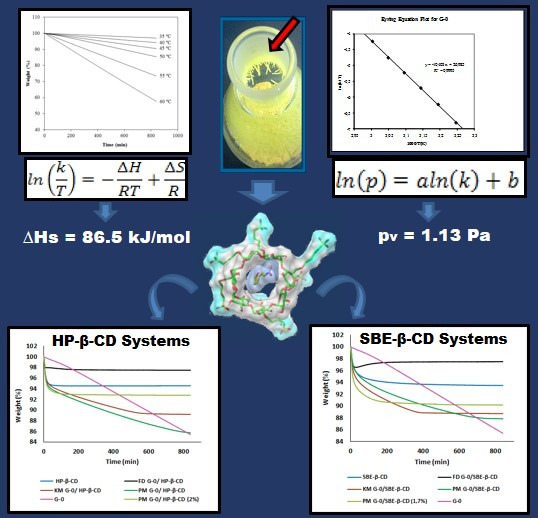
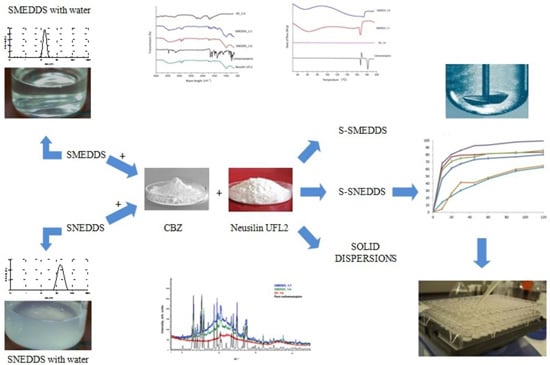
Poorly Soluble Drugs (Closed)
A topical collection in Molecules (ISSN 1420-3049). This collection belongs to the section "Medicinal Chemistry".
Viewed by 363098Editors
Interests: Amorphous solid dispersions, nanocrystals, mesoporous silica, thermal analysis, solid state analysis, spray drying, bead coating
Interests: amorphous drugs and formulations; co-amorphous drug delivery; silica based drug delivery systems; functional excipients
Special Issues, Collections and Topics in MDPI journals
Interests: the solid state of drugs and dosage forms; poorly water soluble drugs; amorphous drugs and drug delivery systems; lipid based drug delivery systems; analytical techniques in the solid state
Interests: freeze-drying and spray-drying; co-amorphous systems; process analytical technology and quality by design; peptides and proteins in pharmaceutical formulations
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
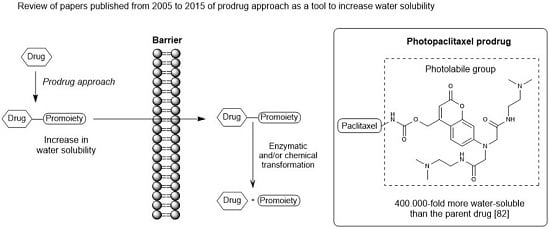
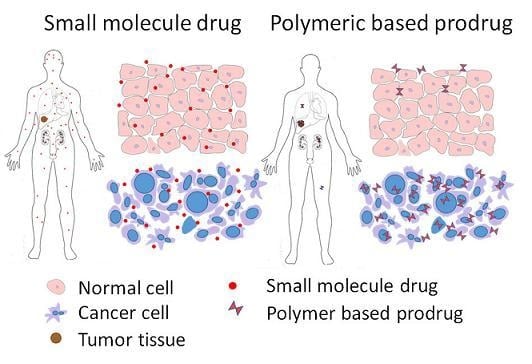
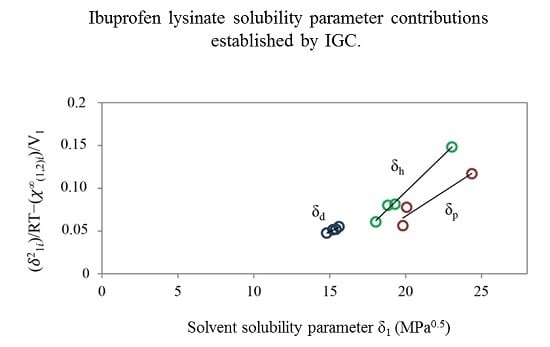
Increasingly important bottlenecks for the development of medicines result from the poor aqueous solubilities and low dissolution rates of many small molecular weight drugs in the pipelines of pharmaceutical companies. To increase the solubilities and dissolution rates of drugs, and thus their bioavailabilities, several feasible approaches can be taken, and are of special interest, both in academia, and in the pharmaceutical industry. These include the conversion of crystalline drugs to their respective amorphous forms, the use of lipid based drug delivery systems, particle size reduction, salt-, co-crystal, and pro-drug formations, and the use of cyclodextrin complexes, to name but a few. This Special Issue aims to provide a forum for the dissemination of the latest information on new approaches and methods for dealing with poorly soluble drugs, and with methods of testing their success.
Prof. Dr. Guy Van den Mooter
Prof. Dr. Korbinian Löbmann
Collection Editors
Prof. Dr. Thomas Rades
Prof. Dr. Holger Grohganz
Former Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molecules is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- solubility
- dissolution rate
- amorphous systems
- lipid based drug delivery systems
- nanoparticles/nanocrystals
- cyclodextrins
- co-crystals
- salts
- prodrugs
- complexes
- supersaturation