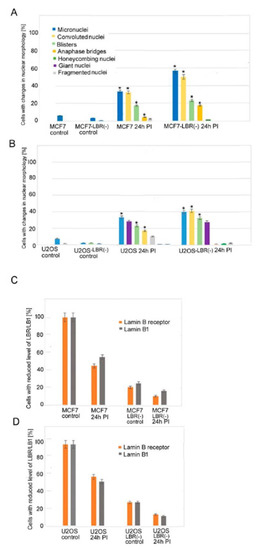
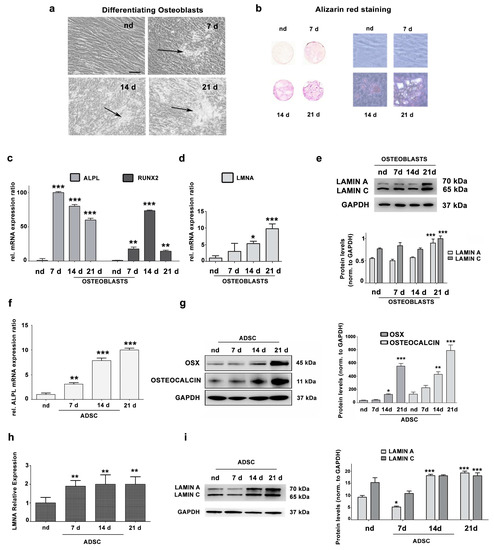
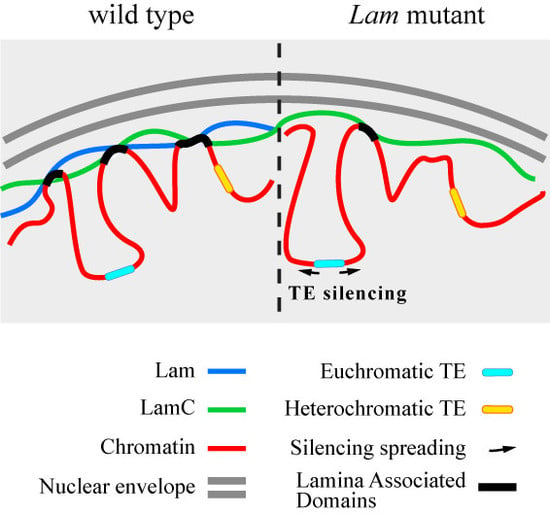
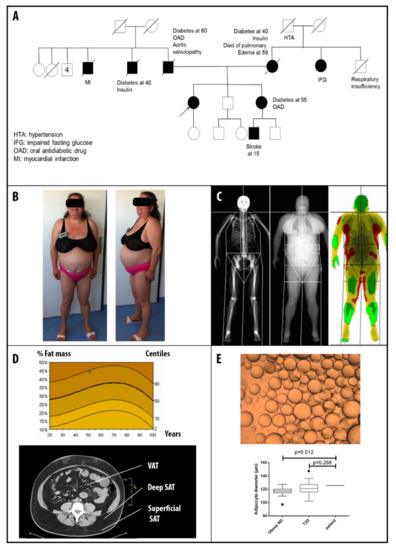
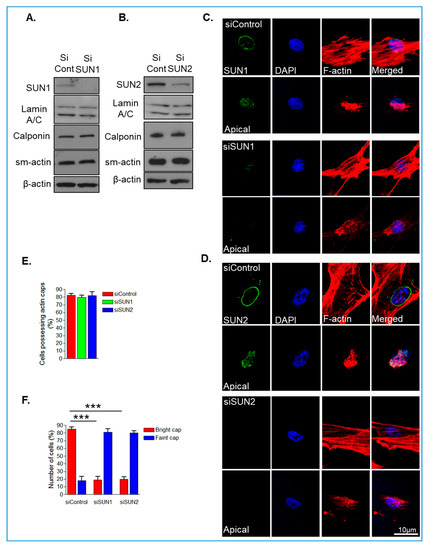
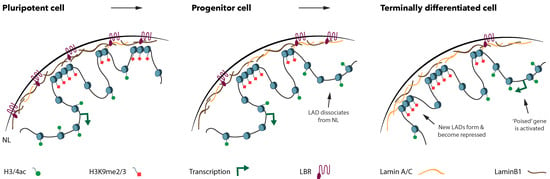
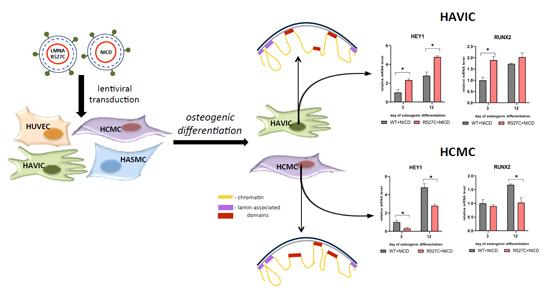
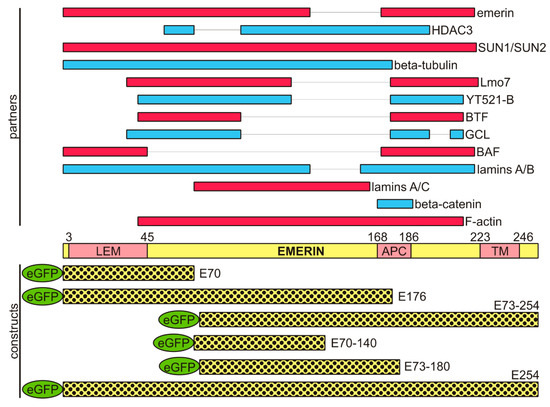
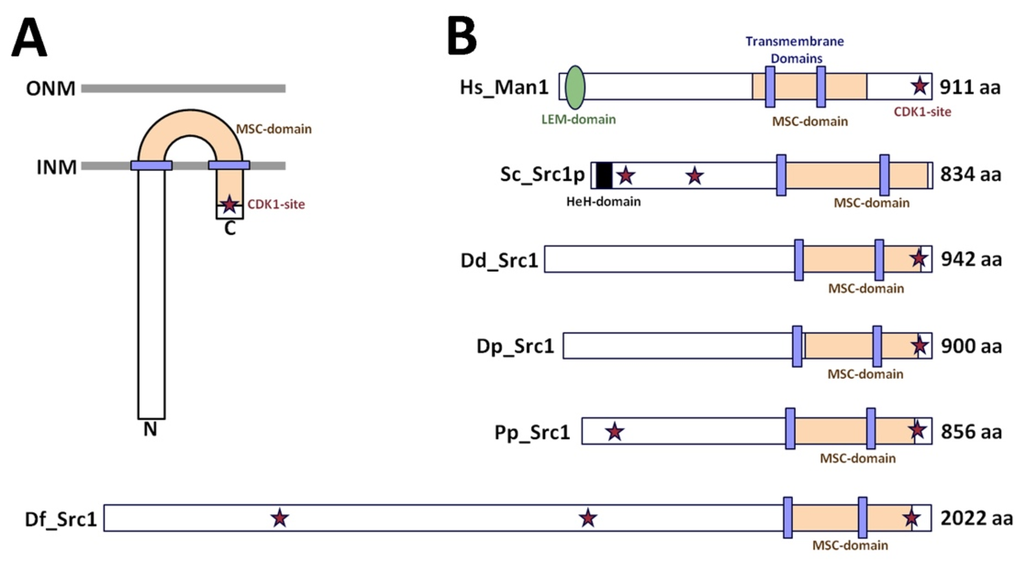
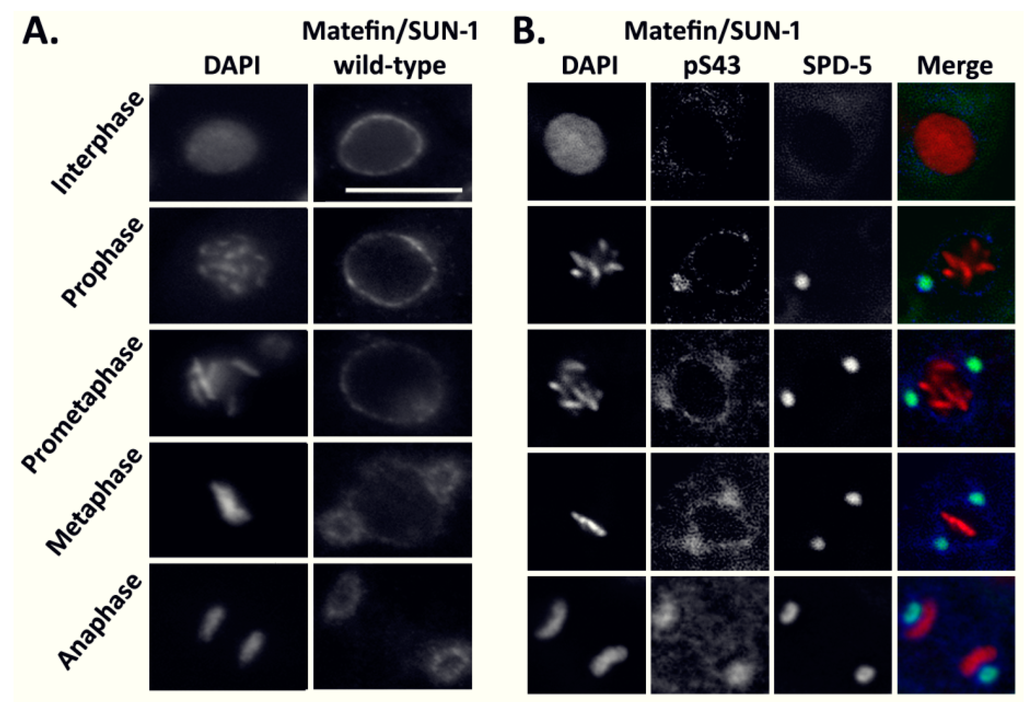
Lamins and Laminopathies
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Nuclei: Function, Transport and Receptors".
Viewed by 362668Editor
Interests: nuclear lamins; LAP2alpha; nuclear structure; nuclear envelope disassembly and assembly; chromatin organization; cell cycle regulation; premature aging; osteogenesis
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
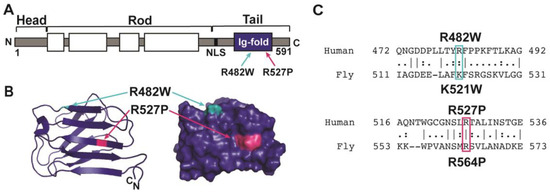
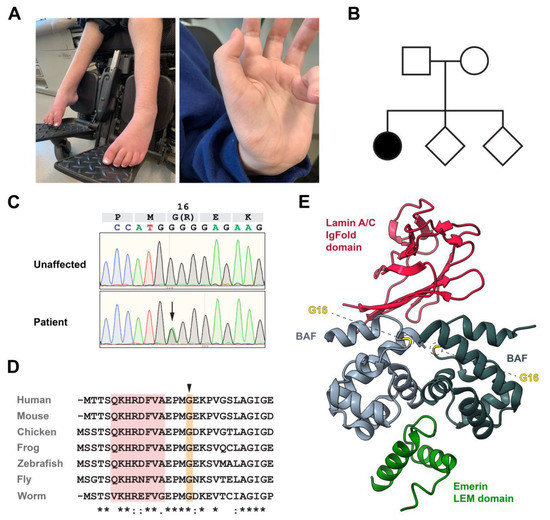
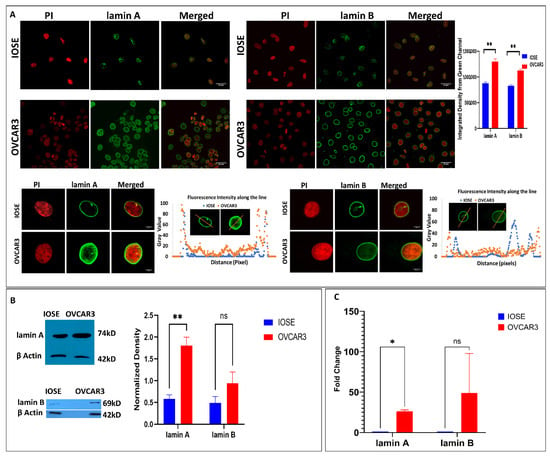
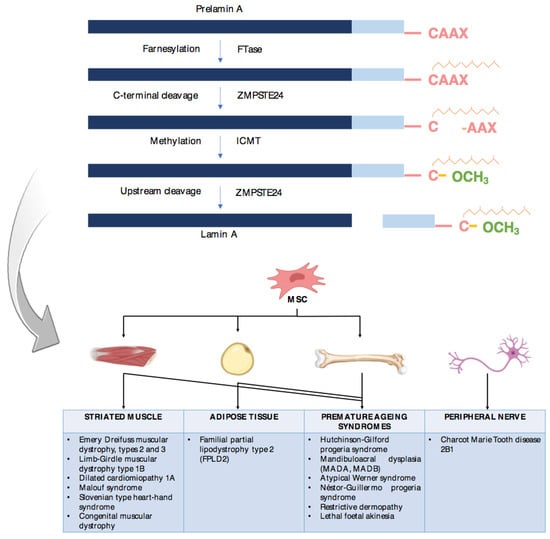
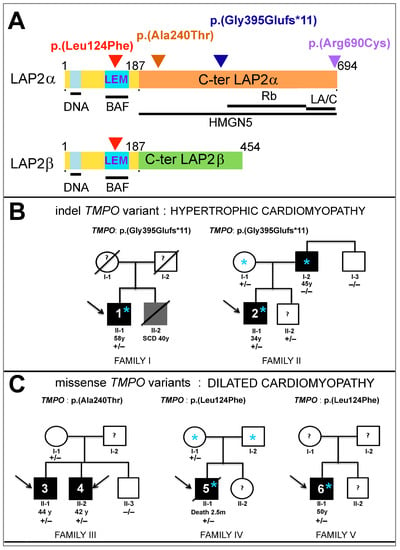
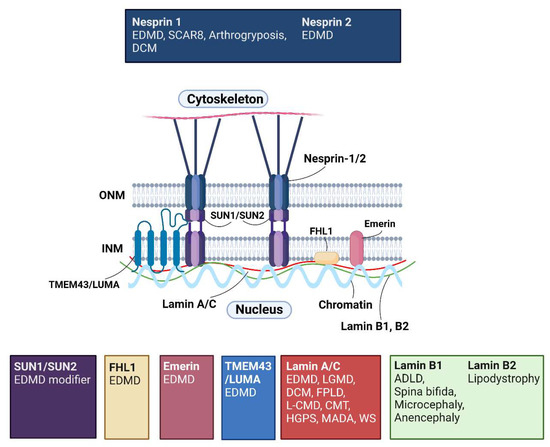
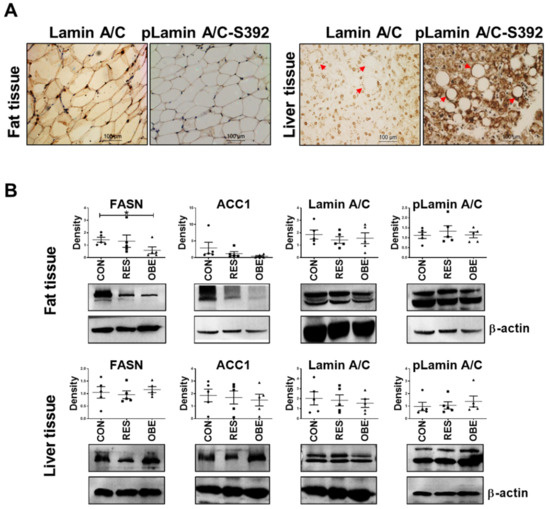
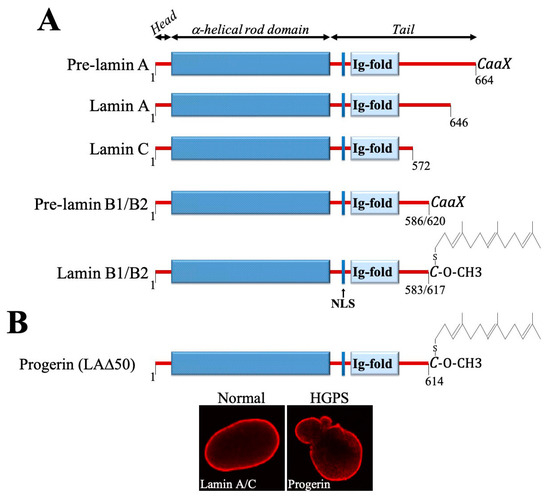
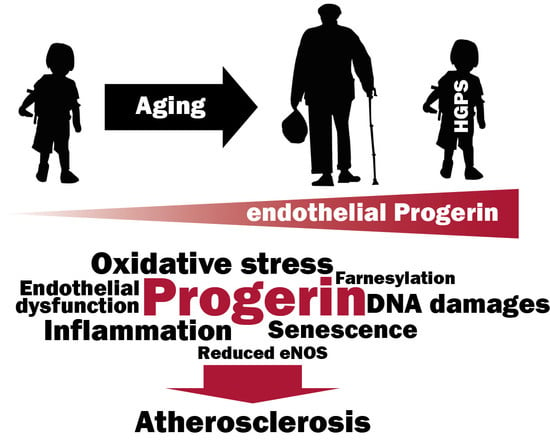
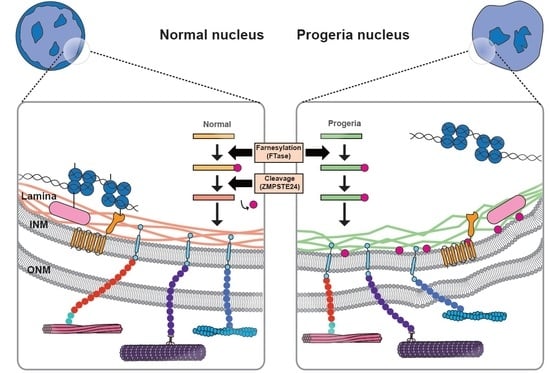
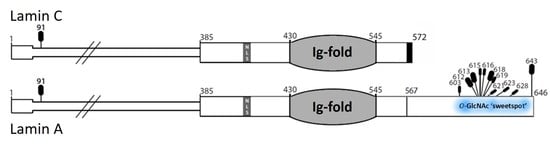
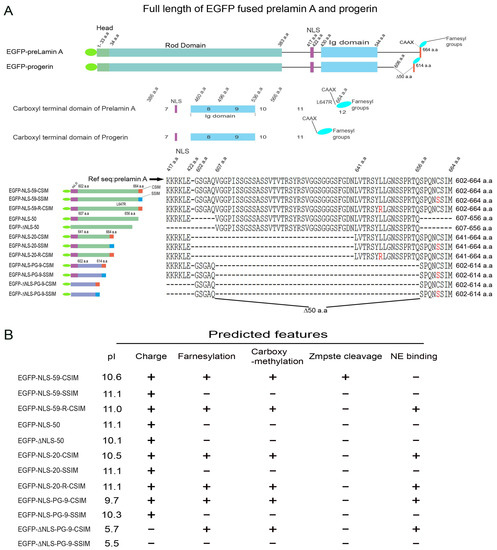
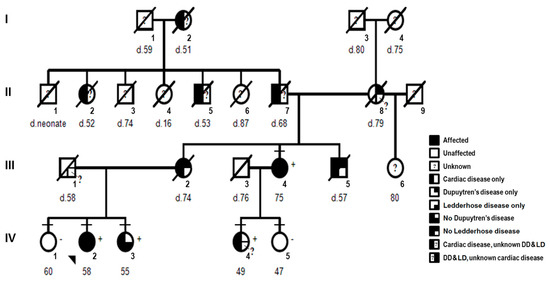
More then 20 years ago, the interest in lamins increased significantly within the scientific community with the discovery that mutations in the gene encoding A-type lamins (LMNA) could lead to human diseases. Up to now there have been reports of nearly 500 distinct mutations in LMNA, associated with over 10 distinct human diseases, which are now generally termed laminopathies and range from muscular dystrophies, cardiomyopathies and lipodystrophies to premature aging diseases, such as Hutchinson-Gilford progeria syndrome.
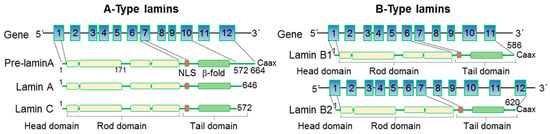
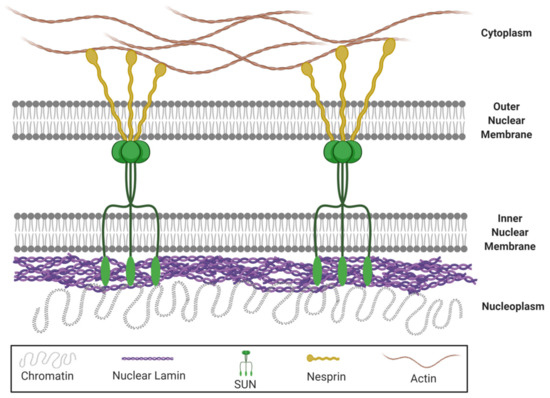
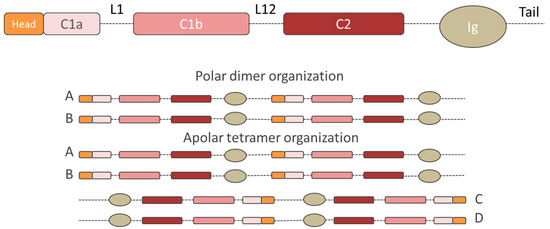
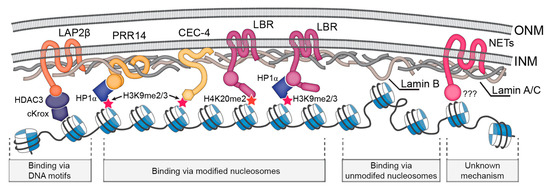
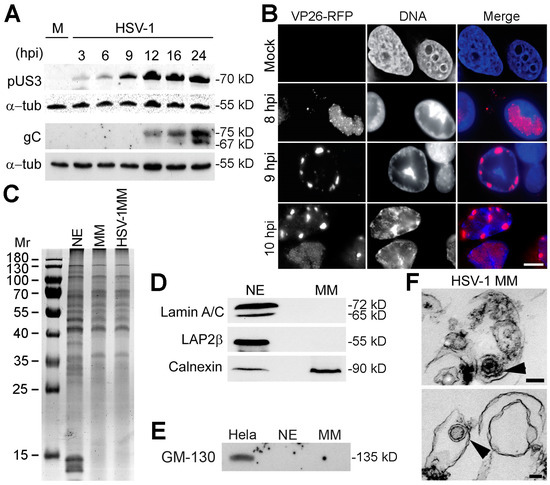
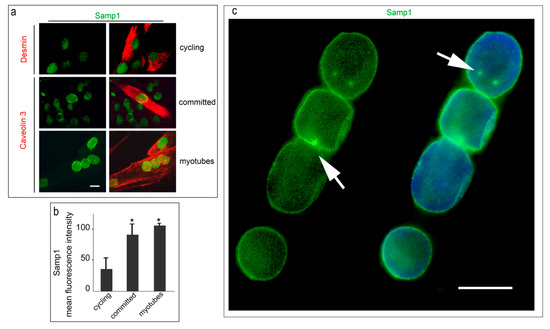
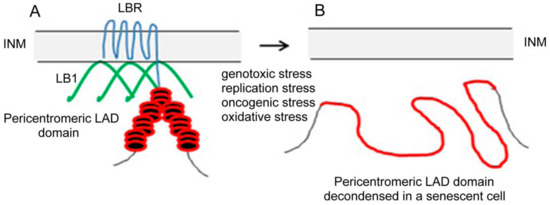
Nuclear lamins are type V intermediate filament (IF) proteins and the main constituents of the nuclear lamina, a filamentous meshwork underlying the inner nuclear membrane and anchoring chromatin to the nuclear envelope (NE). Beside their structural role determining nuclear shape and stability, lamins are involved in various essential cellular functions, such as gene expression, chromatin organization, DNA replication and repair, cell proliferation and differentiation, and mechanosensing. This Topical Collection gives the opportunity to publish original research, as well as review articles, in an Open Access format in the exciting and still growing field of nuclear lamins and laminopathies. We aim to become a platform for lamin researchers and to build a comprehensive collection of articles covering the various aspects of lamins. Therefore we welcome contributions ranging from the molecular structure and properties of lamins, their involvement in specific cellular processes up to the molecular mechanisms underlying the distinct laminopathies in humans and mouse models.
Dr. Thomas Dechat
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- nuclear lamins
- lamin-binding proteins
- chromatin organization
- gene expression
- mechanosensing
- laminopathies
- nuclear envelope
- cell proliferation and differentiation
- DNA repair
- mouse models